VIII. Hypothalamo Pituitary Axis Bone Turnover
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

To Download a List of Prescription Drugs Requiring Prior Authorization
Essential Health Benefits Standard Specialty PA and QL List July 2016 The following products require prior authorization. In addition, there may be quantity limits for these drugs, which is notated below. Therapeutic Category Drug Name Quantity Limit Anti-infectives Antiretrovirals, HIV SELZENTRY (maraviroc) None Cardiology Antilipemic JUXTAPID (lomitapide) 1 tab/day PRALUENT (alirocumab) 2 syringes/28 days REPATHA (evolocumab) 3 syringes/28 days Pulmonary Arterial Hypertension ADCIRCA (tadalafil) 2 tabs/day ADEMPAS (riociguat) 3 tabs/day FLOLAN (epoprostenol) None LETAIRIS (ambrisentan) 1 tab/day OPSUMIT (macitentan) 1 tab/day ORENITRAM (treprostinil diolamine) None REMODULIN (treprostinil) None REVATIO (sildenafil) Soln None REVATIO (sildenafil) Tabs 3 tabs/day TRACLEER (bosentan) 2 tabs/day TYVASO (treprostinil) 1 ampule/day UPTRAVI (selexipag) 2 tabs/day UPTRAVI (selexipag) Pack 2 packs/year VELETRI (epoprostenol) None VENTAVIS (iloprost) 9 ampules/day Central Nervous System Anticonvulsants SABRIL (vigabatrin) pack None Depressant XYREM (sodium oxybate) 3 bottles (540 mL)/30 days Neurotoxins BOTOX (onabotulinumtoxinA) None DYSPORT (abobotulinumtoxinA) None MYOBLOC (rimabotulinumtoxinB) None XEOMIN (incobotulinumtoxinA) None Parkinson's APOKYN (apomorphine) 20 cartridges/30 days Sleep Disorder HETLIOZ (tasimelteon) 1 cap/day Dermatology Alkylating Agents VALCHLOR (mechlorethamine) Gel None Electrolyte & Renal Agents Diuretics KEVEYIS (dichlorphenamide) 4 tabs/day Endocrinology & Metabolism Gonadotropins ELIGARD (leuprolide) 22.5 mg -

Growth Hormone Secretagogues: History, Mechanism of Action and Clinical Development
Growth hormone secretagogues: history, mechanism of action and clinical development Junichi Ishida1, Masakazu Saitoh1, Nicole Ebner1, Jochen Springer1, Stefan D Anker1, Stephan von Haehling 1 , Department of Cardiology and Pneumology, University Medical Center Göttingen, Göttingen, Germany Abstract Growth hormone secretagogues (GHSs) are a generic term to describe compounds which increase growth hormone (GH) release. GHSs include agonists of the growth hormone secretagogue receptor (GHS‐R), whose natural ligand is ghrelin, and agonists of the growth hormone‐releasing hormone receptor (GHRH‐R), to which the growth hormone‐ releasing hormone (GHRH) binds as a native ligand. Several GHSs have been developed with a view to treating or diagnosisg of GH deficiency, which causes growth retardation, gastrointestinal dysfunction and altered body composition, in parallel with extensive research to identify GHRH, GHS‐R and ghrelin. This review will focus on the research history and the pharmacology of each GHS, which reached randomized clinical trials. Furthermore, we will highlight the publicly disclosed clinical trials regarding GHSs. Address for correspondence: Corresponding author: Stephan von Haehling, MD, PhD Department of Cardiology and Pneumology, University Medical Center Göttingen, Göttingen, Germany Robert‐Koch‐Strasse 40, 37075 Göttingen, Germany, Tel: +49 (0) 551 39‐20911, Fax: +49 (0) 551 39‐20918 E‐mail: [email protected]‐goettingen.de Key words: GHRPs, GHSs, Ghrelin, Morelins, Body composition, Growth hormone deficiency, Received 10 September 2018 Accepted 07 November 2018 1. Introduction testing in clinical trials. A vast array of indications of ghrelin receptor agonists has been evaluated including The term growth hormone secretagogues growth retardation, gastrointestinal dysfunction, and (GHSs) embraces compounds that have been developed altered body composition, some of which have received to increase growth hormone (GH) release. -

CS/HB 1041 Controlled Substances SPONSOR(S): Criminal Justice Subcommittee; Berman TIED BILLS: IDEN./SIM
HOUSE OF REPRESENTATIVES STAFF ANALYSIS BILL #: CS/HB 1041 Controlled Substances SPONSOR(S): Criminal Justice Subcommittee; Berman TIED BILLS: IDEN./SIM. BILLS: CS/SB 1448 REFERENCE ACTION ANALYST STAFF DIRECTOR or BUDGET/POLICY CHIEF 1) Criminal Justice Subcommittee 13 Y, 0 N, As Jones Cunningham CS 2) Justice Appropriations Subcommittee 3) Judiciary Committee SUMMARY ANALYSIS Chapter 893, F.S., sets forth the Florida Comprehensive Drug Abuse Prevention and Control Act and classifies controlled substances into five categories, known as schedules. These schedules are used to regulate the manufacture, distribution, preparation and dispensing of the substances listed therein. The distinguishing factors between the different drug schedules are the “potential for abuse” of the substance listed therein and whether there is a currently accepted medical use for the substance. Schedule III substances have a potential for abuse less than the substances contained in schedules I and II, and have an accepted medical use in treatment in the United States. Some examples of schedule III substances are barbituric acid, nalorphine, and anabolic steroids. The bill amends s. 893.03(3), F.S., to add the following substances to schedule III of Florida’s controlled substance schedules: hCG; CJC-1295; GHRH; GHRP-6; HGH; Somatropin; and Tesamorelin. As a result, the criminal penalties relating to the possession, sale, manufacture, delivery, etc. of schedule III controlled substances will apply to these newly added substances. The bill will likely have a negative fiscal impact on the Florida Department of Law Enforcement and the Department of Corrections. See fiscal section. The bill is effective on October 1, 2013. -

Restoring Youth
Restoring Youth The New Science of Human Growth Hormone Therapy Dr. Richard Gaines TABLE OF CONTENTS CHAPI'ER 1 Introduction to Human Growth 1 Hormone Replacement Therapy SECION 1 History of Growth Hormone Use 2 SECION 2 HGH and Hollywood 5 SECION 3 The Benefits of Growth Hormone 8 SECION 4 How HGH Levels are Tested 17 SECION 5 Using Growth Hormone 23 SECION 6 What are the Side Effects? 28 SECION 7 How Much does HGH Cost? 34 SECION 8 Buying Human Growth Hormone 38 SECION 9 Is HGH Legal? 44 CHAPI'ER 2 Related Hormones 47 SECION 1 Testosterone and HGH 48 SECION 2 Thyroid Hormone and HGH 53 SECION 3 Cortisol and Adrenal Fatigue 55 SECION 4 Insulin-like Growth Factor 57 SECION 5 GHRH and Sermorelin 60 SECION 6 Other Hormones and HGH 63 SECION 7 HGH Secretagogues 67 SECION 8 How to Boost HGH Naturally 74 CHAPI'ER 3 HGH Therapy for Men 79 SECION 1 Men and Comprehensive HRT 8o SECION 2 HGH Benefits for Men 86 SECION 3 HGH and Andropause 89 SECION 4 Adrenal Fatigue in Men 94 SECION 5 Men: Is HGH Right for You? 99 CHAPTER 4 HGH Therapy for Women 102 SECION 1 Women and Comprehensive HRT 103 SECION 2 HGH Benefits for Women 110 SECION 3 HGH and Menopause 114 SECION 4 Low Thyroid Syndrome in Women 122 SECION 5 Adrenal Fatigue in Women 128 SECION 6 Women and Bioidentical Hormones 131 SECION 7 Women: Is HGH Right for You? 136 SECION 8 One Woman's Story 139 CHAPTER 5 Choosing the Right HRT Provider 142 SECION 1 Things to Know 143 SECION 2 About HealthGAINS 150 SECION 3 FAQs Frequently Asked Questions 155 CHAPTER 6 Clinical Research 161 SECION 1 What Doctors Say 162 SECION 2 General Health 165 SECION 3 The Brain 168 SECION 4 Weight Loss 171 SECION 5 Anti-Aging 173 SECION 6 Article (Reprint) 175 Low T and Growth Hormone CHAPTER7 Product Information 179 SECION 1 FDA-Approved HGH 180 SECION 2 Illegal HGH 184 SECION 3 Related HGH Hormones 188 SECION 4 Rx HGH Peptides 193 SECION 5 HGH Oral Peptide Secretagogues 196 SECION 6 Homeopathic HGH 199 SECION 7 HGH Supplements 201 Preface THE CONTENT CONTAINED HEREIN IS MEANT TO BE INFORMATIVE ONLY. -

2019 Prohibited List
THE WORLD ANTI-DOPING CODE INTERNATIONAL STANDARD PROHIBITED LIST JANUARY 2019 The official text of the Prohibited List shall be maintained by WADA and shall be published in English and French. In the event of any conflict between the English and French versions, the English version shall prevail. This List shall come into effect on 1 January 2019 SUBSTANCES & METHODS PROHIBITED AT ALL TIMES (IN- AND OUT-OF-COMPETITION) IN ACCORDANCE WITH ARTICLE 4.2.2 OF THE WORLD ANTI-DOPING CODE, ALL PROHIBITED SUBSTANCES SHALL BE CONSIDERED AS “SPECIFIED SUBSTANCES” EXCEPT SUBSTANCES IN CLASSES S1, S2, S4.4, S4.5, S6.A, AND PROHIBITED METHODS M1, M2 AND M3. PROHIBITED SUBSTANCES NON-APPROVED SUBSTANCES Mestanolone; S0 Mesterolone; Any pharmacological substance which is not Metandienone (17β-hydroxy-17α-methylandrosta-1,4-dien- addressed by any of the subsequent sections of the 3-one); List and with no current approval by any governmental Metenolone; regulatory health authority for human therapeutic use Methandriol; (e.g. drugs under pre-clinical or clinical development Methasterone (17β-hydroxy-2α,17α-dimethyl-5α- or discontinued, designer drugs, substances approved androstan-3-one); only for veterinary use) is prohibited at all times. Methyldienolone (17β-hydroxy-17α-methylestra-4,9-dien- 3-one); ANABOLIC AGENTS Methyl-1-testosterone (17β-hydroxy-17α-methyl-5α- S1 androst-1-en-3-one); Anabolic agents are prohibited. Methylnortestosterone (17β-hydroxy-17α-methylestr-4-en- 3-one); 1. ANABOLIC ANDROGENIC STEROIDS (AAS) Methyltestosterone; a. Exogenous* -

Volume 23, Issue 1, Winter 2016/2017
Neuroendocrine & Pituitary Tumor Clinical Center (NEPTCC) Bulletin WINTER 2016/2017 | VOLUME 23 | ISSUE 1 Understanding Growth Hormone Deficiency in HIV Lipodystrophy SUMAN SRINIVASA, MD Perturbations in the growth hormone (GH) axis are common among a significant role in improving metabolic outcomes, related to the well-treated human immunodeficiency virus (HIV)-infected improvements in body composition, dyslipidemia, inflammation, population [1]. Acquired GH deficiency (GHD) secondary to HIV is a cardiovascular and bone health [7-13], which could serve as an clinical diagnosis in the absence of known pituitary or hypothalamic important treatment paradigm in HIV lipodystrophy. pathology and should be differentiated from idiopathic adult Detailed physiology studies have been performed to characterize GH GHD. Studies have determined that a GH peak cutoff of 7.5 ng/mL dynamics in HIV. Overnight frequent sampling among HIV-infected during the GHRH-arginine test can adequately discriminate GHD individuals with and without lipodystrophy and healthy individuals in HIV-infected individuals compared to healthy individuals with demonstrated that the HIV group with lipodystrophy had reduced good specificity. As much as a third of HIV-infected individuals are basal and mean GH concentration, as well as GH pulse amplitude, estimated to have an inappropriate response to GHRH-arginine while GH pulse frequency and IGF-1 levels were normal when stimulation testing [2]. compared to the HIV non-lipodystrophy and healthy control groups A spectrum of disease from true GHD to GH insufficiency exists [14]. Visceral adipose tissue (VAT) accumulation was predictive of among the HIV population. Recent studies have reported that the reduced GH in the HIV population. -
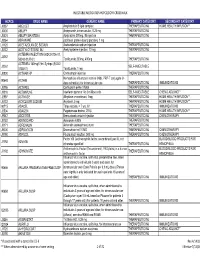
Injectable Medication Hcpcs/Dofr Crosswalk
INJECTABLE MEDICATION HCPCS/DOFR CROSSWALK HCPCS DRUG NAME GENERIC NAME PRIMARY CATEGORY SECONDARY CATEGORY J0287 ABELCET Amphotericin B lipid complex THERAPEUTIC INJ HOME HEALTH/INFUSION** J0400 ABILIFY Aripiprazole, intramuscular, 0.25 mg THERAPEUTIC INJ J0401 ABILIFY MAINTENA Apriprazole 300mg, IM injection THERAPEUTIC INJ J9264 ABRAXANE paclitaxel protein-bound particles, 1 mg J1120 ACETAZOLAMIDE SODIUM Acetazolamide sodium injection THERAPEUTIC INJ J0132 ACETYLCYSTEINE INJ Acetylcysteine injection, 10 mg THERAPEUTIC INJ ACTEMRA INJECTION (50242-0136-01, J3262 50242-0137-01) Tocilizumab 200mg, 400mg THERAPEUTIC INJ ACTEMRA 162mg/0.9ml Syringe (50242- J3262 SELF-INJECTABLE 0138-01) Tocilizumab, 1 mg J0800 ACTHAR HP Corticotropin injection THERAPEUTIC INJ Hemophilus influenza b vaccine (Hib), PRP-T conjugate (4- 90648 ACTHIB dose schedule), for intramuscular use THERAPEUTIC INJ IMMUNIZATIONS J0795 ACTHREL Corticorelin ovine triflutal THERAPEUTIC INJ J9216 ACTIMMUNE Interferon gamma 1-b 3 miillion units SELF-INJECTABLE CHEMO ADJUNCT* J2997 ACTIVASE Alteplase recombinant, 1mg THERAPEUTIC INJ HOME HEALTH/INFUSION** J0133 ACYCLOVIR SODIUM Acyclovir, 5 mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 90715 ADACEL Tdap vaccine, > 7 yrs, IM THERAPEUTIC INJ IMMUNIZATIONS J2504 ADAGEN Pegademase bovine, 25 IU THERAPEUTIC INJ HOME HEALTH/INFUSION** J9042 ADCETRIS Brentuximab vedotin Injection THERAPEUTIC INJ CHEMOTHERAPY J0153 ADENOCARD Adenosine 6 MG THERAPEUTIC INJ J0171 ADRENALIN Adrenalin (epinephrine) inject THERAPEUTIC INJ J9000 ADRIAMYCIN Doxorubicin -

Safety and Metabolic Effects of Tesamorelin, a Growth Hormone-Releasing Factor Analogue, in Patients with Type 2 Diabetes: a Randomized, Placebo-Controlled Trial
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Carolina Digital Repository RESEARCH ARTICLE Safety and metabolic effects of tesamorelin, a growth hormone-releasing factor analogue, in patients with type 2 diabetes: A randomized, placebo-controlled trial David R. Clemmons1*, Sam Miller2, Jean-Claude Mamputu3 1 Division of Endocrinology, Department of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, a1111111111 North Carolina, United States of America, 2 SAM Clinical Research Center, San Antonio, Texas, United States of America, 3 Theratechnologies Inc., Montreal, Quebec, Canada a1111111111 a1111111111 * [email protected] a1111111111 a1111111111 Abstract OPEN ACCESS Objective Citation: Clemmons DR, Miller S, Mamputu J-C Use of growth hormone is associated with side effects, including insulin resistance. The (2017) Safety and metabolic effects of tesamorelin, objective of this study was to determine whether tesamorelin, a stabilized growth hormone- a growth hormone-releasing factor analogue, in patients with type 2 diabetes: A randomized, releasing hormone analogue, would alter insulin sensitivity or control of diabetes. placebo-controlled trial. PLoS ONE 12(6): e0179538. https://doi.org/10.1371/journal. pone.0179538 Design Editor: Noel Christopher Barengo, Florida A 12-week randomized, placebo-controlled study of 53 patients with type 2 diabetes. Three International University Herbert Wertheim College treatment groups: placebo, 1 and 2 mg tesamorelin. of Medicine, UNITED STATES Received: September 7, 2016 Measurements Accepted: May 25, 2017 Fasting glucose, glucose and insulin from oral glucose tolerance test, glycosylated hemo- Published: June 15, 2017 globin (HbA1c), home blood glucose, insulin-like growth factor-1, and lipids. -

Ibm Micromedex® Carenotes Titles by Category
IBM MICROMEDEX® CARENOTES TITLES BY CATEGORY DECEMBER 2019 © Copyright IBM Corporation 2019 All company and product names mentioned are used for identification purposes only and may be trademarks of their respective owners. Table of Contents IBM Micromedex® CareNotes Titles by Category Allergy and Immunology ..................................................................................................................2 Ambulatory.......................................................................................................................................3 Bioterrorism ...................................................................................................................................18 Cardiology......................................................................................................................................18 Critical Care ...................................................................................................................................20 Dental Health .................................................................................................................................22 Dermatology ..................................................................................................................................23 Dietetics .........................................................................................................................................24 Endocrinology & Metabolic Disease ..............................................................................................26 -

Anterior Pituitary Hormones
HYPOTHALAMIC AND ANTERIOR PITUITARY HORMONES Dr. Nisha Department Of Pharmacology HYPOTHALAMIC-PITUITARY RELATIONSHIP HYPOTHALAMIC-PITUITARY RELATIONSHIP . Pituitary gland is connected to hypothalamus by the stalk that contains neurosecretory fibers, capillaries & the hypophyseal portal system that drains the hypothalamus & perfuses the anterior pituitary by a number of releasing factors (RF) or releasing hormones. These hypothalamic releasing factors/hormones stimulate the anterior pituitary to produce & secrete a number of tropic hormones, that in turn stimulate target glands to secrete hormones which finally act on the cells of target organs far away from the site of their release. Pituitary hormone secretions are regulated by negative feedback mechanisms. These pathways are “long” as well as “short” negative feedback pathways. If the hormone secreted affects both the hypothalamus & pituitary, then it makes a long negative feedback loop. If they affect only pituitary, then it is a short negative feedback loop. PITUITARY HORMONES (Overview) GROWTH HORMONE (GH) . GH secretion is high in newborn till 4 yrs of age & starts declining after 25 yrs. Pharmacological Effects of GH: -↑protein synthesis (anabolic) - Positive N2 balance (↑AA uptake in cells) - Anabolic effects are mediated by somatomedins (IGF-1 & IGF-2) - Initially insulin like effects & later anti-insulin effects: ↑blood glucose, ↑FFA mobilization, ketone body formation. - IGF-1 inhibit GH release from ant. pituitary & stimulate GHRIH release from hypothalamus. GH Regulating Factors . GHRH (Growth hormone releasing hormone):- released from hypothalamus, regulate GH release. GHRH analogue: Sermorelin- used as diagnostic agent for testing pituitary GH secretion capability in childhood short stature. GHRIH (Growth hormone release-inhibiting hormone) – Somatostatin It inhibits secretion of GH, TSH, insulin & gastrin. -

INN Working Document 05.179 Update December 2010
INN Working Document 05.179 Update December 2010 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 12/2010 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Essential Medicines and Pharmaceutical Policies (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) © World Health Organization 2010 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

International Nonproprietary Names (Inn) for Biological and Biotechnological Substances
INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 15/06/2006 INTERNATIONAL NONPROPRIETARY NAMES (INN) FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES (A REVIEW) Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines (QSM) Medicines Policy and Standards (PSM) Department CONTENTS 0. INTRODUCTION…………………………………….........................................................................................v 1. PHARMACOLOGICAL CLASSIFICATION OF BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES……………………………………................................1 2. CURRENT STATUS OF EXISTING STEMS OR SYSTEMS FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES…………………….3 2.1 Groups with respective stems ……………………………………………………………………3 2.2 Groups with respective pre-stems………………………………………………………………4 2.3 Groups with INN schemes………………………………………………………………………….4 2.4 Groups without respective stems / pre-stems and without INN schemes…..4 3. GENERAL POLICIES FOR BIOLOGICAL AND BIOTECHNOLOGICAL SUBSTANCES……………………………………………………………………………………………………...5 3.1 General policies for blood products……………………………………………………………5 3.2 General policies for fusion proteins……………………………………………………………5 3.3 General policies for gene therapy products………………………………………………..5 3.4 General policies for glycosylated and non-glycosylated compounds………...6 3.5 General policies for immunoglobulins……………………………………………………….7 3.6 General polices for monoclonal antibodies………………………………………………..7 3.7 General polices for skin substitutes……………………………………………………………9 3.8 General policies for transgenic products……………………………………………………9