Download Exhibitor List (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oleds and E-PAPER Disruptive Potential for the European Display Industry
OLEDs AND E-PAPER Disruptive potential for the European display industry Authors: Simon Forge and Colin Blackman Editor: Sven Lindmark EUR 23989 EN - 2009 The mission of the JRC-IPTS is to provide customer-driven support to the EU policy- making process by developing science-based responses to policy challenges that have both a socio-economic as well as a scientific/technological dimension. European Commission Joint Research Centre Institute for Prospective Technological Studies Contact information Address: Edificio Expo. c/ Inca Garcilaso, 3. E-41092 Seville (Spain) E-mail: [email protected] Tel.: +34 954488318 Fax: +34 954488300 http://ipts.jrc.ec.europa.eu http://www.jrc.ec.europa.eu Legal Notice Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use which might be made of this publication. Europe Direct is a service to help you find answers to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server http://europa.eu/ JRC 51739 EUR 23989 EN ISBN 978-92-79-13421-0 ISSN 1018-5593 DOI 10.2791/28548 Luxembourg: Office for Official Publications of the European Communities © European Communities, 2009 Reproduction is authorised provided the source is acknowledged Printed in Spain PREFACE Information and Communication Technology (ICT) markets are exposed to a more rapid cycle of innovation and obsolescence than most other industries. -

Technology and Services Solutions Guide
Insights Report | March 2020 COVID-19 TECHNOLOGY AND SERVICES SOLUTIONS GUIDE To our provider friends: You need solutions and you need them fast. We have asked the vendor community to share what they are doing/can do to help your organizations in this crisis. Given the short time frame, KLAS has not validated any of these claims. We will not waste time with a long introduction—we just hope this helps you. Good luck in the weeks ahead. Reach out to your KLAS representative if we can help you. Thank you for bearing the weight of this crisis. We deeply appreciate your sacrifices. —The KLAS Team 1 Table of Contents 3 Technology Solutions 4 Ambulatory EHR Vendors 114 Medical Equipment 10 Ambulatory RCM Services 116 Patient Access 12 Analytics 120 Patient Engagement 20 Behavioral and Public Health 136 Patient Financial Experience 25 Capacity Management 139 Patient Intake Management 36 Clinical Decision Support & Surveillance 142 Patient Outreach 47 COVID-19 Organizational 153 Payer Solutions Readiness Assessment 159 Pharmacy Surveillance & Infusion Therapy 50 COVID-19 Provider Profile & 162 Population Health Management Communication Platform 193 Post–Acute Care 52 COVID-19 Research Collaboration & Patient-Data Networks 199 Remote Education & Training 59 COVID-19 Screening, Tracking, & 203 Remote Patient Monitoring Care Management 211 Remote Work Capabilities & Security 66 Emergency & Urgent Care 215 Revenue Cycle Management 68 Enterprise EHR Vendors 222 RTLS & Medical Device Management 75 Identity & Access Management 233 Scheduling 78 Imaging & Oncology 238 Secure Communication 93 Infection Control & Monitoring 244 Security & Privacy 99 Interactive Patient Systems 251 Speech Recognition 104 Internal Communication 255 Supply Chain/ERP 108 Interoperability Platforms 259 Telehealth/Virtual Care 112 Laboratory Solutions 280 Services 344 Vendor/Firm Index Report Information Copyright Infringement Warning This report and its contents are copyright-protected works and are intended solely for your organization. -

Master Thesis Master's Programme in Industrial Management and Innovation, 120 Credits
Master Thesis Master's Programme in Industrial Management and Innovation, 120 credits Capturing Business Model Innovation Driven by the Emergence of New Technologies in Established Firms A Case Study at Siemens Healthineers Thesis in Industrial Innovation Management, 30 credits Halmstad 2018-06-16 Emma Bäckman, Josefin Ellmarker HALMSTAD UNIVERSITY Abstract Background: It is argued that the emergence of new technologies and the digitalization can improve the healthcare, make it more efficient, personalized and available for everyone. The healthcare has already begun to become more digitized and there is no doubt that this trend will continue. Moreover, it is argued that AI will have a major impact on the MedTech and healthcare industry. Problem discussion: To stay competitive it has been concluded that firms must update and rethink their business models constantly thus, to undertake business model innovation. This applies specially to established firms that have been successful with the same business model for a long time. Despite the fact that the existing literature addresses the importance of business model innovation, very little attention has been drawn to how to actually achieve this change. Purpose: The purpose of this study is to explore how business models in established firms within the MedTech industry develops over time due to the emergence of new technologies. More specifically, we focus on how artificial intelligence is influencing and will influence the business models in the MedTech industry. Methodology: This study has been performed through a single case study at Siemens Healthineers. The primary data has been gathered through four meetings with people with expertise in the field of artificial intelligence as well as the MedTech and healthcare industry. -

Ess School 9-800-143 Rev
Harvard Business School 9-800-143 Rev. May 18, 2000 E Ink This is a chance for all of us to leave a legacy. Nothing I’ve seen has shaken my belief that this technology is just fundamentally revolutionary. —Jim Iuliano, President & CEO of E Ink The research building of E Ink in Cambridge, Massachusetts, sounded more like a party than a lab for serious, cutting-edge technology. Loud polka music and the laughter of young employees filled the air as they went about their work. Founded just over a year earlier, in 1997, the company aimed to revolutionize print communication through display technology. Black and white photographs, hung throughout the lab, captured scenes of everyday expression that could be affected by E Ink’s technology; these photos included everything from subway graffiti to the sign for the tobacco shop in Harvard Square. Across the parking lot, a different building housed the offices of E Ink’s management team, including Jim Iuliano, president and CEO of E Ink, and Russ Wilcox, vice president and general manager. A prototype of E Ink’s first product hung outside their offices—a sign prepared for JC Penney. It read, “Reebok High Tops on sale today. Sale ends Friday.” Electronic ink (e-ink) was an ink solution, composed of tiny paint particles and dye, that could be activated by an electric charge. This ink could be painted onto nearly any type of surface— including thin, flexible plastics. Charged particles could display one color, such as white, while the dye displayed another color, such as blue. -

Medical Additive Manufacturing/ 3D Printing Annual Report 2018 Improving Public Health
Medical Additive Manufacturing/ 3D Printing Annual Report 2018 Improving Public Health Each year, healthcare needs and costs grow due to an aging population, the rise in chronic diseases, and more. In fact, global healthcare spending is projected to reach nearly $9 trillion by 20201. To address this, practitioners in the healthcare industry continue to look for innovations that can provide quality care to patients at a reasonable cost. But they can’t do it alone. Today, the manufacturing industry is an important partner, with one particularly bright opportunity focused on Medical Additive Manufacturing/3D Printing (AM3DP). From anatomical models to early bioprinting applications, the use of AM3DP is providing benefits for patients and physicians/institutions including: ■ Better patient outcomes ■ Less time in the operating room ■ Reduced costs In 2017, as outlined in this Annual Report, collaboration between hospitals, device manufacturers, U.S. Food and Drug Administration (FDA), and partners such as SME, led to extraordinary strides in identifying industry trends, opportunities, challenges and solutions. These partnerships drive efficiency through best practice sharing as well as accelerate innovation for applications such as bioprinting and tissue fabrication. They also lay the groundwork for 3D printing of organs and scaling up production of tissues which are still decades away. With millions of patients already directly impacted by the technology, this momentum continues into 2018 and beyond where AM3DP will continue to positively impact public health and drive strong business results. This 2017 Annual Report covers: COVER: Justin Ryan holds a pediatric heart model 3D-printed at the ■ Industry Overview Phoenix Children’s Hospital Cardiac 3D Print Lab. -

Exhibitors' Forum Schedule
p47-55 Exhibitor Forum_Layout 1 4/23/2019 9:51 AM Page 47 Exhibitors’ Forum Schedule TuesDay, May 14, execuTive BallrooM session F1: Display Design anD ManuFacTuring 11:00 am – 12:45 pm F1.1: enabling Display innovations Through new Developments in open (11:00) industry standards Craig Wiley, Video Electronics Standards Association (VESA), San Jose, CA Booth 641 The Video Electronics Standards Association (VESA) will present new advancements in VESA display standards that push resolutions beyond 8K and enable life-like AR/VR. Also featured are high-dynamic-range (HDR) certification fFo1r .m2: o n iptoirxse iln-gclruaddineg lOoLcEaDl aDnidm nmotienbgo ofoksr, Hhiigghhe rD dyinspalmayi icn rtearfnagce c o m p r e s s i o n r a t e s , a n d n e w e f f o r t s (fo1r1 h:1ig5h)- r e s o l u t i o Mn ianugt Cohmeont,i BveO Edi Tspeclahynso. logy Group Co, Ltd., Beijing, China Booth 808 An ultra-high-definition display incorporating high dynamic range (HDR) and 5G content-delivery provides a great viewing experience. This presentation will introduce the HDR technology trend and describe future requirements for display devices to fulfill the HDR standard. Black Diamond is a technology that uses two LCD cells together to achieve a pixel-grade local-dimming approach that will highly improve the contrast detail for LCD devices. AF1 co.3m: p a lriasomni wnaillt iboen m aaduet oamoantgio cnu rarenndt minatiengstrraetaimon H D R t e c h n o l o g i e s , i n c l u d i n g B l a c k - D i a m o n d , m i n i L E (D1, 1O:L3E0D) , e t c . -

Healthcare & Life Sciences Group
HEALTHCARE & LIFE SCIENCES GROUP 2 1 Healthcare and life sciences clients have long turned to S&C for help succeeding in today’s rapidly changing business environment. Large and mid-size, public and private, throughout their lifecycles, these companies rely on our multi-disciplinary, global team to address their most complex legal and business challenges and reach their strategic goals. Sector expertise: We offer unrivaled OUR CLIENTS GET… knowledge of the healthcare and life sciences industries, our clients’ businesses and the sector-specific competitive pressures bearing down on them. Sullivan & Cromwell’s Healthcare and Life Sciences Group has negotiated complex transactions and resolved high-stakes disputes for almost three decades. Today, it possesses an unparalleled grasp of these sectors and a practical understanding of the commercial realities underlying them. We position our clients to succeed through it all. The Firm represents international clients in the following healthcare sectors: Pharmaceuticals and Life Sciences Medtech Health Insurers Healthcare Services 2 Legal expertise: Clients come to us An integrated, global team: for the high-quality counsel and hands-on We’re a core group of dedicated healthcare representation we offer across multiple advisers across our 13 offices on four legal specialties, to successfully execute continents with a strong track record of their most important deals and resolve the sector’s most significant transactions critical disputes. We can execute any type and litigation matters, supported by all of transaction in any economic climate or the resources of an integrated, global firm. geographic region. Our experience in this We’re grateful to our clients for trusting sector includes: us with their future, and we’ll continue to help them position themselves for growth M&A and success in this exciting and ever- Corporate finance changing industry. -

Conference Programme Speakers Overview
CONFERENCE BOOK Celebrating 60 years of the benefits of innovation in medical technology to Society CONFERENCE PROGRAMME & SPEAKERS OVERVIEW 13 & 14 NOVEMBER 2019 COCIR, the European Coordination Committee of the Radiological, Electromedical and Healthcare IT Industry European Coordination Committee of the Radiological, Electromedical and Healthcare IT Industry PROGRAM WEDNESDAY 13 NOVEMBER 2019 17:30 REGISTRATION & WELCOME Drinks, Photographs 18:30 ANNIVERSARY CEREMONY OPENING BY COCIR PRESIDENT Jan KIMPEN / COCIR President and Chief Medical Officer at Royal Philips KEYNOTE SPEECHES BY EUROPEAN COMMISSION Manuel MATEO GOYET / Member of the Cabinet of Commissioner Gabriel, the Digital Economy and Society portfolio at the European Commission Andrzej RYS / Director Health Systems, Medical Products & Innovation at DG Santé, European Commission HEALTHCARE FUTUROLOGIST: “WHAT DOES THE FUTURE HOLD?” Koen KAS / Healthcare futurist, entrepreneur, professor of molecular oncology, and renowned international keynote speaker. 19:30 GALA DINNER Museum visit (optional) & Networking 2 COCIR 60 YEARS ANNIVERSARY COCIR PROGRAM PROGRAM THURSDAY 14 NOVEMBER 2019 08:30 REGISTRATION & WELCOME / Coffee 09:00 WELCOME by Nicole DENJOY / COCIR Secretary General Moderation by Maryline FIASCHI / Managing Director, Science Business 09:15 KEYNOTE SPEECH BY FINNISH PRESIDENCY Tuula HELANDER / Senior Advisor, Cabinet and Strategy Group of the Permanent Secretary, Ministry of Social Affairs and Health, Health Secretary General, Finnish Cancer Institute 09:30 SESSION -

E-Paper Technology
Special Issue - 2016 International Journal of Engineering Research & Technology (IJERT) ISSN: 2278-0181 NSDMCC - 2015 Conference Proceedings E-Paper Technology Anitta Joseph Vth Semester B.Sc. Computer Science Vimala College, Thrissur Abstract: Made of flexible material, requiring ultra-low These limitations include the backlighting of monitors power consumption, cheap to manufacture, and most which is hard on the human eye, while electronic paper importantly, easy and convenient to read, E-papers of the reflects light just like normal paper. In addition, e-paper is future are just around the corner, with the promise to hold easier to read at an angle than flat screen monitors. libraries on a chip and replace most printed newspapers Electronic paper also has the potential to be flexible before the end of the next decade.Electronic paper(E-paper) is a portable. Reusable storage and display medium that looks becauseit is made of plastic. It is also light and potentially like paper but can be repeatedly written on (refreshed) by inexpensive. electronic means, thousands or millions of times. E-paper will be used for applications such as e-books, electronics II. TECHNOLOGY BEHIND E_PAPER newspaper, portable signs, & foldable, rollable displays. Information to be displays is downloaded through a The E-Paper is also called Electronic Paper or Electronic connection to a computer or a cell phone, or created with ink Display. The first E-Paper was developed in 1974’s by mechanical tools such as an electronic “pencil”. This paper Nicholas K Sheridon at Xerox’s Palo Alto research centre. discusses the history, features, and technology of the electronic paper revolution. -

Common Stocks — 104.5%
Eaton Vance Tax-Advantaged Global Dividend Income Fund January 31, 2021 PORTFOLIO OF INVESTMENTS (Unaudited) Common Stocks — 104.5% Security Shares Value Aerospace & Defense — 0.8% Safran S.A.(1) 98,721 $ 12,409,977 $ 12,409,977 Banks — 6.7% Bank of New York Mellon Corp. (The) 518,654 $ 20,657,989 Citigroup, Inc. 301,884 17,506,253 HDFC Bank, Ltd.(1) 512,073 9,775,702 ING Groep NV(1) 1,676,061 14,902,461 Japan Post Bank Co., Ltd. 445,438 3,851,696 Mitsubishi UFJ Financial Group, Inc. 2,506,237 11,317,609 Mizuho Financial Group, Inc. 292,522 3,856,120 Sumitomo Mitsui Financial Group, Inc. 186,747 5,801,916 Wells Fargo & Co. 341,979 10,218,332 $ 97,888,078 Beverages — 1.0% Diageo PLC 378,117 $ 15,180,328 $ 15,180,328 Biotechnology — 1.2% CSL, Ltd. 82,845 $ 17,175,550 $ 17,175,550 Building Products — 0.9% Assa Abloy AB, Class B 509,607 $ 12,603,485 $ 12,603,485 Chemicals — 0.7% Sika AG 38,393 $ 10,447,185 $ 10,447,185 Construction & Engineering — 0.0% Abengoa S.A., Class A(1)(2) 311,491 $ 0 Abengoa S.A., Class B(1)(2) 3,220,895 0 $0 Construction Materials — 0.9% CRH PLC 332,889 $ 13,660,033 $ 13,660,033 Consumer Finance — 0.6% Capital One Financial Corp. 79,722 $ 8,311,816 $ 8,311,816 1 Security Shares Value Diversified Financial Services — 2.5% Berkshire Hathaway, Inc., Class B(1) 101,853 $ 23,209,243 ORIX Corp. -
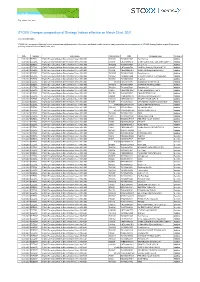
STOXX Changes Composition of Strategy Indices Effective on March 22Nd, 2021
Zug, March 13th, 2021 STOXX Changes composition of Strategy Indices effective on March 22nd, 2021 Dear Sir and Madam, STOXX Ltd., the operator of Qontigo’s index business and a global provider of innovative and tradable index concepts, today announced the new composition of STOXX Strategy Indices as part of the regular quarterly review effective on March 22nd, 2021 Date Symbol Index name Internal Key ISIN Company name Changes 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW01UK TW0001722007 Twn Fertilizer Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD CN0010 CNE1000002H1 CHINA CONSTRUCTION BANK CORP H Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW02MD TW0002301009 Liteon Tech Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD CN249E CNE000000T59 YANTAI CHANGYU PION.WINE 'B' Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD CN1I6O CNE100000HF9 CHINA MINSHENG BANKING H Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW025W TW0002015005 Feng Hsin Iron Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW505Z TW0006412000 CHICONY POWER TECHNOLOGY Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW0751 TW0004938006 Pegatron Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD 691316 HK0270001396 Guangdong Investment Ltd. Addition 12.03.2021 EDEDSL STOXX Emerging Markets -

Siemens Annual Report 2018
Annual Report 2018 siemens.com Table of contents . A B C Combined Management Report Consolidated Financial Statements Additional Information A.1 p 2 B.1 p 62 C.1 p 132 Organization of the Siemens Group Consolidated Statements Responsibility Statement and basis of presentation of Income C.2 p 133 A.2 p 3 B.2 p 63 Independent Auditor ʼs Report Financial performance system Consolidated Statements of Comprehensive Income C.3 p 139 A.3 p 6 Report of the Supervisory Board Segment information B.3 p 64 Consolidated Statements C.4 p 144 A.4 p 18 of Financial Position Corporate Governance Results of operations B.4 p 65 C.5 p 157 A.5 p 21 Consolidated Statements Notes and forward- looking Net assets position of Cash Flows statements B.5 p 66 A.6 p 22 Financial position Consolidated Statements of Changes in Equity A.7 p 26 B.6 p 68 Overall assessment of the economic position Notes to Consolidated Financial Statements A.8 p 28 Report on expected developments and associated material opportunities and risks A.9 p 40 Siemens AG A.10 p 43 Compensation Report A.11 p 57 Takeover-relevant information A. Combined Management Report A.1 Organization of the Siemens Group and basis of pr esentation Siemens is a technology company with core activities in the fields Non-financial matters of the Group of electrification, automation and digitalization and activities and Siemens AG in nearly all countries of the world. We are a leading supplier of Siemens has policies for environmental, employee and social power generation, power transmission and infrastructure solu- matters, for the respect of human rights, and anti-corruption and tions as well as automation, drive and software solutions for in- bribery matters, among others.