Healthcare & Life Sciences Group
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Symbols Alexian Brothers Health System Index to Nov P
Volume 19, Index December 2014 Symbols Alexian Brothers Health System Index to Nov p. 2 Health Care M&A News 1-800-CONTACTS Feb p. 18 A&L Goodbody Apr p. 16, July p. 16 All Care Home Health, LLC Feb p. 17 Each company and orga- A Allergan Inc. Feb p. 10, May p. 1, nization discussed in Health May p. 19, Jun p. 15, July p. 3, Care M&A News, from Janu- Abbott Labs July p. 3, Aug p. 17, July p. 10, Sept p. 16, Oct p. 3, ary through December 2014, Dec p. 4 Nov p. 1, Dec p. 4, Dec p. 16, is listed alphabetically here. AbbVie Inc. July p. 3, July p. 16, Dec p. 19 References are typically to Aug p. 9, Oct p. 2, Oct p. 16, Allscripts Feb p. 2, Apr p. 10 the first occurrence of the Nov p. 19, Dec p. 2 AllSpire Health Partners Nov p. 2 company’s or organiza- ABL, Inc. Aug p. 18 All Staffing Services Jan p. 17 tion’s name in the pertinent Acadia Healthcare Company Feb p. 16, Almost Family, Inc. Apr p. 16 issue; further discussion July p. 17, Oct p. 17, Nov p. 17 ALN Medical Management, LLC may follow later in the text Accelera Innovations, Inc. Jan p. 17 Accelrys, Inc. Jan p. 15 Feb p. 8 but is not indicated here. AccessClosure, Inc. May p. 15 Alta Partners Jun p. 16 Achillion Pharmaceuticals July p. 17 Altos Solutions, Inc. Jun p. 15 References are sorted by Actavis (Foshan) Pharmaceuticals Co. -

Master Thesis Master's Programme in Industrial Management and Innovation, 120 Credits
Master Thesis Master's Programme in Industrial Management and Innovation, 120 credits Capturing Business Model Innovation Driven by the Emergence of New Technologies in Established Firms A Case Study at Siemens Healthineers Thesis in Industrial Innovation Management, 30 credits Halmstad 2018-06-16 Emma Bäckman, Josefin Ellmarker HALMSTAD UNIVERSITY Abstract Background: It is argued that the emergence of new technologies and the digitalization can improve the healthcare, make it more efficient, personalized and available for everyone. The healthcare has already begun to become more digitized and there is no doubt that this trend will continue. Moreover, it is argued that AI will have a major impact on the MedTech and healthcare industry. Problem discussion: To stay competitive it has been concluded that firms must update and rethink their business models constantly thus, to undertake business model innovation. This applies specially to established firms that have been successful with the same business model for a long time. Despite the fact that the existing literature addresses the importance of business model innovation, very little attention has been drawn to how to actually achieve this change. Purpose: The purpose of this study is to explore how business models in established firms within the MedTech industry develops over time due to the emergence of new technologies. More specifically, we focus on how artificial intelligence is influencing and will influence the business models in the MedTech industry. Methodology: This study has been performed through a single case study at Siemens Healthineers. The primary data has been gathered through four meetings with people with expertise in the field of artificial intelligence as well as the MedTech and healthcare industry. -

Preclinical Development & IND Filing: Nuts, Bolts, Best Practices
“Preclinical Development & IND Filing: Nuts, Bolts, Best Practices and Regulatory Aspects.” Speakers: Amit Kalgutkar (Pfizer), Chandra Prakash (Agios), Sanjeev Thohan (Novartis), Li- Chun Wang (Takeda), Wei Yin (Biogen) Organizers: Sanjeev Thohan and Chandra Prakash Date: 6/29/2017 Time: 8:30 am – 5.00 pm Location: Boston/Cambridge Area: Marriott Kendall Square, 50 Broadway, Cambridge MA 02142 Fees: $175 - Regular (before June 1st, 2017), $225 - Regular (after June 1st, 2017); $125 - Unemployed & Academic; $2000 - Major Sponsorship; $475 - Vendor Show Registration: www.PBSS.org Workshop Description: An investigational new drug application (IND) is an important milestone that marks the entry of a molecule into clinical development. Knowing the objectives, expectations and processes of assembling an IND is a key to not only successful filing, but also a promising clinical development path forward. Often there are cases where too many of our “nice-to-have” studies crowd the package at the expense of critical study needs/issues. This can lead to significant delays in clinical developments with back-and-forth of Q&A sessions both internally and with regulatory agencies. As we have seen, the regulatory landscape is changing as rapidly as the industry innovates into new therapeutic modalities. Therefore, it is critical to keep up to date on regulatory requirements and the industry’s best practices in different aspects of the IND: non- clinical safety, PK, CMC, and clinical plans. In this workshop, our speakers who bring years of experience with multiple successful IND filings, will discuss systematically the preclinical studies required for small molecule IND’s as well as the nuts and bolts of putting together a high–quality IND package. -

Medical Additive Manufacturing/ 3D Printing Annual Report 2018 Improving Public Health
Medical Additive Manufacturing/ 3D Printing Annual Report 2018 Improving Public Health Each year, healthcare needs and costs grow due to an aging population, the rise in chronic diseases, and more. In fact, global healthcare spending is projected to reach nearly $9 trillion by 20201. To address this, practitioners in the healthcare industry continue to look for innovations that can provide quality care to patients at a reasonable cost. But they can’t do it alone. Today, the manufacturing industry is an important partner, with one particularly bright opportunity focused on Medical Additive Manufacturing/3D Printing (AM3DP). From anatomical models to early bioprinting applications, the use of AM3DP is providing benefits for patients and physicians/institutions including: ■ Better patient outcomes ■ Less time in the operating room ■ Reduced costs In 2017, as outlined in this Annual Report, collaboration between hospitals, device manufacturers, U.S. Food and Drug Administration (FDA), and partners such as SME, led to extraordinary strides in identifying industry trends, opportunities, challenges and solutions. These partnerships drive efficiency through best practice sharing as well as accelerate innovation for applications such as bioprinting and tissue fabrication. They also lay the groundwork for 3D printing of organs and scaling up production of tissues which are still decades away. With millions of patients already directly impacted by the technology, this momentum continues into 2018 and beyond where AM3DP will continue to positively impact public health and drive strong business results. This 2017 Annual Report covers: COVER: Justin Ryan holds a pediatric heart model 3D-printed at the ■ Industry Overview Phoenix Children’s Hospital Cardiac 3D Print Lab. -

Conference Programme Speakers Overview
CONFERENCE BOOK Celebrating 60 years of the benefits of innovation in medical technology to Society CONFERENCE PROGRAMME & SPEAKERS OVERVIEW 13 & 14 NOVEMBER 2019 COCIR, the European Coordination Committee of the Radiological, Electromedical and Healthcare IT Industry European Coordination Committee of the Radiological, Electromedical and Healthcare IT Industry PROGRAM WEDNESDAY 13 NOVEMBER 2019 17:30 REGISTRATION & WELCOME Drinks, Photographs 18:30 ANNIVERSARY CEREMONY OPENING BY COCIR PRESIDENT Jan KIMPEN / COCIR President and Chief Medical Officer at Royal Philips KEYNOTE SPEECHES BY EUROPEAN COMMISSION Manuel MATEO GOYET / Member of the Cabinet of Commissioner Gabriel, the Digital Economy and Society portfolio at the European Commission Andrzej RYS / Director Health Systems, Medical Products & Innovation at DG Santé, European Commission HEALTHCARE FUTUROLOGIST: “WHAT DOES THE FUTURE HOLD?” Koen KAS / Healthcare futurist, entrepreneur, professor of molecular oncology, and renowned international keynote speaker. 19:30 GALA DINNER Museum visit (optional) & Networking 2 COCIR 60 YEARS ANNIVERSARY COCIR PROGRAM PROGRAM THURSDAY 14 NOVEMBER 2019 08:30 REGISTRATION & WELCOME / Coffee 09:00 WELCOME by Nicole DENJOY / COCIR Secretary General Moderation by Maryline FIASCHI / Managing Director, Science Business 09:15 KEYNOTE SPEECH BY FINNISH PRESIDENCY Tuula HELANDER / Senior Advisor, Cabinet and Strategy Group of the Permanent Secretary, Ministry of Social Affairs and Health, Health Secretary General, Finnish Cancer Institute 09:30 SESSION -

Common Stocks — 104.5%
Eaton Vance Tax-Advantaged Global Dividend Income Fund January 31, 2021 PORTFOLIO OF INVESTMENTS (Unaudited) Common Stocks — 104.5% Security Shares Value Aerospace & Defense — 0.8% Safran S.A.(1) 98,721 $ 12,409,977 $ 12,409,977 Banks — 6.7% Bank of New York Mellon Corp. (The) 518,654 $ 20,657,989 Citigroup, Inc. 301,884 17,506,253 HDFC Bank, Ltd.(1) 512,073 9,775,702 ING Groep NV(1) 1,676,061 14,902,461 Japan Post Bank Co., Ltd. 445,438 3,851,696 Mitsubishi UFJ Financial Group, Inc. 2,506,237 11,317,609 Mizuho Financial Group, Inc. 292,522 3,856,120 Sumitomo Mitsui Financial Group, Inc. 186,747 5,801,916 Wells Fargo & Co. 341,979 10,218,332 $ 97,888,078 Beverages — 1.0% Diageo PLC 378,117 $ 15,180,328 $ 15,180,328 Biotechnology — 1.2% CSL, Ltd. 82,845 $ 17,175,550 $ 17,175,550 Building Products — 0.9% Assa Abloy AB, Class B 509,607 $ 12,603,485 $ 12,603,485 Chemicals — 0.7% Sika AG 38,393 $ 10,447,185 $ 10,447,185 Construction & Engineering — 0.0% Abengoa S.A., Class A(1)(2) 311,491 $ 0 Abengoa S.A., Class B(1)(2) 3,220,895 0 $0 Construction Materials — 0.9% CRH PLC 332,889 $ 13,660,033 $ 13,660,033 Consumer Finance — 0.6% Capital One Financial Corp. 79,722 $ 8,311,816 $ 8,311,816 1 Security Shares Value Diversified Financial Services — 2.5% Berkshire Hathaway, Inc., Class B(1) 101,853 $ 23,209,243 ORIX Corp. -
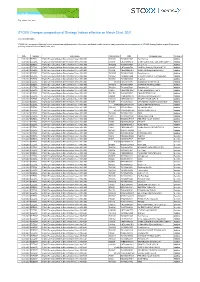
STOXX Changes Composition of Strategy Indices Effective on March 22Nd, 2021
Zug, March 13th, 2021 STOXX Changes composition of Strategy Indices effective on March 22nd, 2021 Dear Sir and Madam, STOXX Ltd., the operator of Qontigo’s index business and a global provider of innovative and tradable index concepts, today announced the new composition of STOXX Strategy Indices as part of the regular quarterly review effective on March 22nd, 2021 Date Symbol Index name Internal Key ISIN Company name Changes 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW01UK TW0001722007 Twn Fertilizer Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD CN0010 CNE1000002H1 CHINA CONSTRUCTION BANK CORP H Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW02MD TW0002301009 Liteon Tech Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD CN249E CNE000000T59 YANTAI CHANGYU PION.WINE 'B' Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD CN1I6O CNE100000HF9 CHINA MINSHENG BANKING H Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW025W TW0002015005 Feng Hsin Iron Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW505Z TW0006412000 CHICONY POWER TECHNOLOGY Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD TW0751 TW0004938006 Pegatron Addition 12.03.2021 EDEDSL STOXX Emerging Markets Diversification Select 100 USD 691316 HK0270001396 Guangdong Investment Ltd. Addition 12.03.2021 EDEDSL STOXX Emerging Markets -

Siemens Annual Report 2018
Annual Report 2018 siemens.com Table of contents . A B C Combined Management Report Consolidated Financial Statements Additional Information A.1 p 2 B.1 p 62 C.1 p 132 Organization of the Siemens Group Consolidated Statements Responsibility Statement and basis of presentation of Income C.2 p 133 A.2 p 3 B.2 p 63 Independent Auditor ʼs Report Financial performance system Consolidated Statements of Comprehensive Income C.3 p 139 A.3 p 6 Report of the Supervisory Board Segment information B.3 p 64 Consolidated Statements C.4 p 144 A.4 p 18 of Financial Position Corporate Governance Results of operations B.4 p 65 C.5 p 157 A.5 p 21 Consolidated Statements Notes and forward- looking Net assets position of Cash Flows statements B.5 p 66 A.6 p 22 Financial position Consolidated Statements of Changes in Equity A.7 p 26 B.6 p 68 Overall assessment of the economic position Notes to Consolidated Financial Statements A.8 p 28 Report on expected developments and associated material opportunities and risks A.9 p 40 Siemens AG A.10 p 43 Compensation Report A.11 p 57 Takeover-relevant information A. Combined Management Report A.1 Organization of the Siemens Group and basis of pr esentation Siemens is a technology company with core activities in the fields Non-financial matters of the Group of electrification, automation and digitalization and activities and Siemens AG in nearly all countries of the world. We are a leading supplier of Siemens has policies for environmental, employee and social power generation, power transmission and infrastructure solu- matters, for the respect of human rights, and anti-corruption and tions as well as automation, drive and software solutions for in- bribery matters, among others. -

Siemens Healthineers · Flyer DIN Long Portrait · Template
Dr. Roland Busch Information on the Supervisory Board candidate proposed for election under Agenda Item 6 2002 Siemens Aktiengesellschaft, Head of Memberships of comparable domestic or Infotainment Solutions Division foreign controlling bodies of commercial enterprises: 2005 Siemens VDO Automotive Asia Pacific Co. Ltd., Shanghai, China • Atos SE, Bezons, France • President and CEO • Arabia Electric Ltd. (Equipment), Jeddah, * 2007 Siemens Aktiengesellschaft, Saudi Arabia Transportation Systems Group, • ESMT European School of Management Erlangen, Germany and Technology GmbH, Berlin, Germany • Head of Mass Transit Division Dr. Roland Busch • ISCOSA Industries and Maintenance Ltd., * 2008 Siemens Aktiengesellschaft, Dammam, Saudi Arabia (deputy chairman) Deputy Chief Executive Officer, Chief Corporate Development Department, • Siemens Ltd., Riyadh, Saudi Arabia* Technology Officer and member of the Munich, Germany Managing Board of Siemens Aktien • Siemens W.L.L., Doha, Qatar* gesellschaft, Berlin and Munich, Germany • Head of Corporate Strategies • VA TECH T&D Co. Ltd., Riyadh, born on November 22, 1964 in Erlangen, 2011 Member of the Managing Board of Saudi Arabia* Siemens Aktiengesellschaft Germany • Chief Technology Officer since Dr. Busch is a member of the Managing December 2016 Education: Board of Siemens Aktiengesellschaft, with its • Chief Operating Officer from registered seat in Berlin and Munich. Siemens October 2018 until September 2019 • Studied physics at Friedrich Alexander since Aktiengesellschaft directly and indirectly University in ErlangenNuremberg, Germany, 2019 • Chairman of the Supervisory Board holds 85% of the Company’s capital stock at and at the University of Grenoble, France of Siemens Mobility GmbH since the time Notice of the Annual Shareholders’ Meeting is given. Pursuant to Section 5.4.1 (6) • Dipl.Phys., Dr. -

Life Sciences Newsletter Q2 2018
KPMG Corporate Finance LLC Healthcare Life Sciences M&AQuarterly Q2 2018 © 2018 KPMG Corporate Finance LLC, a Delaware limited liability company. Member FINRA and SIPC. KPMG Corporate Finance LLC is a subsidiary of KPMG LLP, a Delaware limited liability partnership and the U.S. member firm of the KPMG network of independent member firms affiliated with KPMG International Cooperative (“KPMG International”), a Swiss entity. All rights reserved. LIFE SCIENCES M&A NEWSLETTER KPMG Corporate Finance LLC Global Life Sciences Sector Coverage(1) U.S. Life Sciences Team Jason Moran Amanda Dydynski Erik Jordan Sandy Prabhakar Managing Director & Head Senior Associate Director & Head of Director & Head of of Healthcare Life Sciences Healthcare Life Sciences Private Equity International Desk San Francisco San Francisco Los Angeles Chicago T: 415-949-8909 T: 415-963-8401 T: 213-955-8722 T: 312-665-1990 E: [email protected] E: [email protected] E: [email protected] E: [email protected] United Kingdom Germany France Canada Japan Andrew Nicholson Florian Frei Guillaume Cauchoix Neil Blair Toshiaki Ishii Ireland Sweden Spain India China Michele Connolly Christian Terslow Luis Zaragoza Sanjay Singh Andy Qiu Finland Switzerland Italy South Korea Australia Jukka Teikari Patrik Kerler Paulo Mascaretti Sung-Won Park Peter Turner Netherlands Austria Portugal Vietnam New Zealand Danny Bosker Klaus Mittermair João Leal John Ditty Gary Ivory Denmark Belgium Poland Russia Philippines Eric Bots-Bjerre Peter Lauwers Krzysztof Klamut Robert Vartevanian Michael Guarin KPMG Corporate Finance(1) KPMG Corporate Finance provides a broad range of investment banking and strategic advisory services to domestic and international clients and is a leading global middle market investment banking platform — Average 350 transactions annually for the last five years — Over 2,500 investment banking professionals worldwide — Offices in 84 countries with extensive cross-border abilities Notes: (1) Represents the global Corporate Finance practices of KPMG International’s network of independent member firms. -

Stamford Therapeutics Consortium Consortiapedia.Fastercures.Org
Stamford Therapeutics Consortium consortiapedia.fastercures.org Stamford Therapeutics Consortium consortiapedia.fastercures.org/consortia/stamford-therapeutics-consortium/ Research Areas At a Glance Status: Active Consortium Tool Development Year Launched: 1994 Clinical Trial Initiating Organization: Dr. Paul Dalgin Data-Sharing Enabler Initiator Type: Industry Location: North America DevelopmentProduct Drugs Abstract Stamford Therapeutics Consortium (STC) is a privately owned and operated clinical research site specializing in phase II, III, and IV clinical trials for the pharmaceutical and biotechnology industries. STC has a strong working relationship with a cardiology group, a large multispecialty medical practice, and a team of physicians who serve as sub-investigators for many of its clinical trials. STC maintains a significant presence in research for the treatment of osteoarthritis and rheumatoid arthritis, while continuing to expand its research into new therapeutic areas. Year after year STC's list of specialties grows to accommodate a growing demand and new innovations in healthcare. With over 15 years of clinical trials operations, STC has conducted more than 350 national and multi-national clinical trials. Mission The company’s sole mission is to conduct the highest quality clinical trials so that new, safe, and effective medications can be developed, researched, and approved for a variety of indications and diseases. Stamford Therapeutics Consortium - consortiapedia.fastercures.org Page 1/4 Stamford Therapeutics Consortium consortiapedia.fastercures.org Consortium History The site was launched in 1994 and has contributed to clinical trials research ever since. Structure & Governance The consortium is governed by their president, medical director, founder, and clinical research coordinators. Patent Engagement STC actively recruits patients from all over Fairfield County, CT and Westchester County, NY. -

Bristol-Myers Squibb Company [email protected]
UNITED STATES SECURITIES A ND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 DIVISION OF CORPORATION FINANCE March 1, 2019 Lisa A. Atkins Bristol-Myers Squibb Company [email protected] Re: Bristol-Myers Squibb Company Incoming letter dated December 21, 2018 Dear Ms. Atkins: This letter is in response to your correspondence dated December 21, 2018 concerning the shareholder proposal (the “Proposal”) submitted to Bristol-Myers Squibb Company (the “Company”) by People for the Ethical Treatment of Animals (the “Proponent”) for inclusion in the Company’s proxy materials for its upcoming annual meeting of security holders. We also have received correspondence from the Proponent dated January 3, 2019. Copies of all of the correspondence on which this response is based will be made available on our website at http://www.sec.gov/divisions/corpfin/ cf-noaction/14a-8.shtml. For your reference, a brief discussion of the Division’s informal procedures regarding shareholder proposals is also available at the same website address. Sincerely, M. Hughes Bates Special Counsel Enclosure cc: Jared Goodman PETA Foundation [email protected] March 1, 2019 Response of the Office of Chief Counsel Division of Corporation Finance Re: Bristol-Myers Squibb Company Incoming letter dated December 21, 2018 The Proposal asks the board to implement a policy that it will not fund, conduct or commission use of the “Forced Swim Test.” There appears to be some basis for your view that the Company may exclude the Proposal under rule 14a-8(i)(7), as relating to the Company’s ordinary business operations. In our view, the Proposal micromanages the Company by seeking to impose specific methods for implementing complex policies.