Chromosomal Abnormalities Associated with Omphalocele
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Monosomy X Turner Syndrome Information for Patients
Monosomy X Turner syndrome Information for patients The healthcare professional responsible for your care has given you this leaflet because you have been identified by the Harmony® Prenatal Test as having a high probability of a chromosome disorder in your pregnancy. This fact sheet contains more information about the particular genetic disorder mentioned in your Harmony report. We recommend that you also discuss your result with an experienced doctor or genetic counsellor. Turner syndrome, or Monosomy X, is a sex chromosome disorder that occurs in females when there is only one copy of the X chromosome instead of the expected two (Figure 1). It occurs in at least one in every 2,500 female births. Monosomy X may be associated with an increased risk of miscarriage in the first or second trimester. More than half of those withT urner syndrome will be mosaic, meaning some of their cells have just one X chromosome and the other cells have two X chromosomes. Features and symptoms of Turner syndrome include subtle changes in physical appearance, short stature, infertility and learning difficulties, as well as some potential health conditions, including cardiac conditions, hypothyroidism, diabetes and autoimmune disease. Babies who are born with Turner syndrome could have a number of the features and symptoms of the syndrome, however, not everyone will have them all and severity will vary significantly. Mosaicism also plays a role in the varied severity of the syndrome. Although there is no cure for Turner syndrome, many of the associated symptoms can be treated. Girls with Turner syndrome may need regular health checks of their heart, kidneys and reproductive system throughout their lives. -

Chromosome 18
Chromosome 18 Description Humans normally have 46 chromosomes in each cell, divided into 23 pairs. Two copies of chromosome 18, one copy inherited from each parent, form one of the pairs. Chromosome 18 spans about 78 million DNA building blocks (base pairs) and represents approximately 2.5 percent of the total DNA in cells. Identifying genes on each chromosome is an active area of genetic research. Because researchers use different approaches to predict the number of genes on each chromosome, the estimated number of genes varies. Chromosome 18 likely contains 200 to 300 genes that provide instructions for making proteins. These proteins perform a variety of different roles in the body. Health Conditions Related to Chromosomal Changes The following chromosomal conditions are associated with changes in the structure or number of copies of chromosome 18. Distal 18q deletion syndrome Distal 18q deletion syndrome occurs when a piece of the long (q) arm of chromosome 18 is missing. The term "distal" means that the missing piece (deletion) occurs near one end of the chromosome arm. The signs and symptoms of distal 18q deletion syndrome include delayed development and learning disabilities, short stature, weak muscle tone ( hypotonia), foot abnormalities, and a wide variety of other features. The deletion that causes distal 18q deletion syndrome can occur anywhere between a region called 18q21 and the end of the chromosome. The size of the deletion varies among affected individuals. The signs and symptoms of distal 18q deletion syndrome are thought to be related to the loss of multiple genes from this part of the long arm of chromosome 18. -

Classic and Molecular Cytogenetic Analyses Reveal Chromosomal Gains and Losses Correlated with Survival in Head and Neck Cancer Patients
Vol. 11, 621–631, January 15, 2005 Clinical Cancer Research 621 Classic and Molecular Cytogenetic Analyses Reveal Chromosomal Gains and Losses Correlated with Survival in Head and Neck Cancer Patients Na´dia Aparecida Be´rgamo,1 that acquisition of monosomy 17 was a significant (P = Luciana Caricati da Silva Veiga,1 0.0012) factor for patients with a previous family history of Patricia Pintor dos Reis,4 Ineˆs Nobuko Nishimoto,3 cancer. Conclusions: The significant associations found in this Jose´ Magrin,3 Luiz Paulo Kowalski,3 4 2 study emphasize that alterations of distinct regions of the Jeremy A. Squire, and Sı´lvia Regina Rogatto genome may be genetic biomarkers for a poor prognosis. 1Department of Genetics, Institute of Biosciences and 2NeoGene Losses of chromosomes 17 and 22 can be associated with Laboratory, Department of Urology, Faculty of Medicine, Sa˜o Paulo a family history of cancer. State University; 3Department of Head and Neck Surgery and Otorhinolaryngology, AC Camargo Hospital, Sa˜o Paulo, Brazil and 4Department of Cellular and Molecular Biology, Princess Margaret INTRODUCTION Hospital, Ontario Cancer Institute, University of Toronto, Toronto, Carcinomas of the head and neck represent the sixth most Ontario, Canada frequent cancer worldwide and f90% to 95% are squamous cell carcinomas. Tobacco and alcohol consumption are the ABSTRACT most important nongenetic risk factors associated with the Purpose: Genetic biomarkers of head and neck tumors development of head and neck squamous cell carcinomas could be useful for distinguishing among patients with (HNSCC; ref. 1). Estimated age-standardized rates per similar clinical and histopathologic characteristics but 100,000 for 1990 showed 12.8 men and 3.7 women of oral having differential probabilities of survival. -

Abstracts from the 50Th European Society of Human Genetics Conference: Electronic Posters
European Journal of Human Genetics (2019) 26:820–1023 https://doi.org/10.1038/s41431-018-0248-6 ABSTRACT Abstracts from the 50th European Society of Human Genetics Conference: Electronic Posters Copenhagen, Denmark, May 27–30, 2017 Published online: 1 October 2018 © European Society of Human Genetics 2018 The ESHG 2017 marks the 50th Anniversary of the first ESHG Conference which took place in Copenhagen in 1967. Additional information about the event may be found on the conference website: https://2017.eshg.org/ Sponsorship: Publication of this supplement is sponsored by the European Society of Human Genetics. All authors were asked to address any potential bias in their abstract and to declare any competing financial interests. These disclosures are listed at the end of each abstract. Contributions of up to EUR 10 000 (ten thousand euros, or equivalent value in kind) per year per company are considered "modest". Contributions above EUR 10 000 per year are considered "significant". 1234567890();,: 1234567890();,: E-P01 Reproductive Genetics/Prenatal and fetal echocardiography. The molecular karyotyping Genetics revealed a gain in 8p11.22-p23.1 region with a size of 27.2 Mb containing 122 OMIM gene and a loss in 8p23.1- E-P01.02 p23.3 region with a size of 6.8 Mb containing 15 OMIM Prenatal diagnosis in a case of 8p inverted gene. The findings were correlated with 8p inverted dupli- duplication deletion syndrome cation deletion syndrome. Conclusion: Our study empha- sizes the importance of using additional molecular O¨. Kırbıyık, K. M. Erdog˘an, O¨.O¨zer Kaya, B. O¨zyılmaz, cytogenetic methods in clinical follow-up of complex Y. -

Cytogenetics, Chromosomal Genetics
Cytogenetics Chromosomal Genetics Sophie Dahoun Service de Génétique Médicale, HUG Geneva, Switzerland [email protected] Training Course in Sexual and Reproductive Health Research Geneva 2010 Cytogenetics is the branch of genetics that correlates the structure, number, and behaviour of chromosomes with heredity and diseases Conventional cytogenetics Molecular cytogenetics Molecular Biology I. Karyotype Definition Chromosomal Banding Resolution limits Nomenclature The metaphasic chromosome telomeres p arm q arm G-banded Human Karyotype Tjio & Levan 1956 Karyotype: The characterization of the chromosomal complement of an individual's cell, including number, form, and size of the chromosomes. A photomicrograph of chromosomes arranged according to a standard classification. A chromosome banding pattern is comprised of alternating light and dark stripes, or bands, that appear along its length after being stained with a dye. A unique banding pattern is used to identify each chromosome Chromosome banding techniques and staining Giemsa has become the most commonly used stain in cytogenetic analysis. Most G-banding techniques require pretreating the chromosomes with a proteolytic enzyme such as trypsin. G- banding preferentially stains the regions of DNA that are rich in adenine and thymine. R-banding involves pretreating cells with a hot salt solution that denatures DNA that is rich in adenine and thymine. The chromosomes are then stained with Giemsa. C-banding stains areas of heterochromatin, which are tightly packed and contain -

Congenital Heart Disease and Chromossomopathies Detected By
Review Article DOI: 10.1590/0103-0582201432213213 Congenital heart disease and chromossomopathies detected by the karyotype Cardiopatias congênitas e cromossomopatias detectadas por meio do cariótipo Cardiopatías congénitas y anomalías cromosómicas detectadas mediante cariotipo Patrícia Trevisan1, Rafael Fabiano M. Rosa2, Dayane Bohn Koshiyama1, Tatiana Diehl Zen1, Giorgio Adriano Paskulin1, Paulo Ricardo G. Zen1 ABSTRACT Conclusions: Despite all the progress made in recent de- cades in the field of cytogenetic, the karyotype remains an es- Objective: To review the relationship between congenital sential tool in order to evaluate patients with congenital heart heart defects and chromosomal abnormalities detected by disease. The detailed dysmorphological physical examination the karyotype. is of great importance to indicate the need of a karyotype. Data sources: Scientific articles were searched in MED- LINE database, using the descriptors “karyotype” OR Key-words: heart defects, congenital; karyotype; Down “chromosomal” OR “chromosome” AND “heart defects, syndrome; trisomy; chromosome aberrations. congenital”. The research was limited to articles published in English from 1980 on. RESUMO Data synthesis: Congenital heart disease is characterized by an etiologically heterogeneous and not well understood Objetivo: Realizar uma revisão da literatura sobre a group of lesions. Several researchers have evaluated the pres- relação das cardiopatias congênitas com anormalidades ence of chromosomal abnormalities detected by the karyo- cromossômicas detectadas por meio do exame de cariótipo. type in patients with congenital heart disease. However, Fontes de dados: Pesquisaram-se artigos científicos no most of the articles were retrospective studies developed in portal MEDLINE, utilizando-se os descritores “karyotype” Europe and only some of the studied patients had a karyo- OR “chromosomal” OR “chromosome” AND “heart defects, type exam. -

Cell Biology
Course Code-EDU161 and Course Name:Botany Aneuploidy: Meaning, Forms and Importance | Cell Biology Meaning of Aneuploidy: Aneuploidy is the presence of chromosome number that is different from the simple multiple of the basic chromosome number. An organism which contains one or more incomplete chromosome sets is known as aneuploid. Aneuploidy can be either due to loss of one or more chromosomes (hypo-ploidy) or due to addition of one or more chromosomes to complete chromosome complement (hyper-ploidy). Hypo-ploidy may be due to loss of a single chromosome – monosomy (2n – 1), or due to loss of one pair of chromosomes – nullisomy (2n – 2). Similarly, hyper-ploidy may involve addition of either a single chromosome-trisomy (2n + 1) or a pair of chromosomes (2n + 2) – tetrasomy (Fig. 11.2). Forms of Aneuploidy: Monosomy: Monosomy is the phenomenon where an individual lacks one or a few non-homologous chromosome(s) of a diploid complement Types of Monosomy: Single monosomies lack one complete chromosome (2n – 1), these create major imbalance and may not be tolerated in diploids. Monosomies, however, are viable in polyploid species, in which the loss of one chromosome has a less marked effect. For instance, in ordinary tobacco, Nicotiana tabacum, which is a tetraploid species with 2n = 48 chromosomes, a series of different monosomic types with 47 chromosomes is known. The number of possible monosomies in an organism is equal to the haploid chromosome number. Double monosomies (2n – 1 – 1) or triple monosomies (2n – 1 – 1 – 1) could also be produced in polyploids. Origin of Monosomy: The origin of the monosomies may be from the production of n – 1 types of gametes due to rare nondisjunction of a bivalent. -
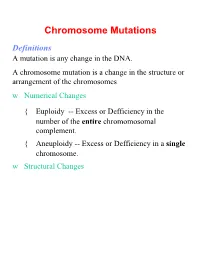
Chromosome Mutations Definitions a Mutation Is Any Change in the DNA
Chromosome Mutations Definitions A mutation is any change in the DNA. A chromosome mutation is a change in the structure or arrangement of the chromosomes w Numerical Changes { Euploidy -- Excess or Defficiency in the number of the entire chromomosomal complement. { Aneuploidy -- Excess or Defficiency in a single chromosome. w Structural Changes Numerical Chromosome Mutations Euploid -- “True Number” The cell(s) have the same number of copies of all the chromosome. 1. Monoploid (Haploid if a gamete) 2. Diploid (Often is not considered a Euploid) 3. Triploid 4. Tetraploid Euploidy Chromosomes shown at metaphase Normal Diploid Euploid Monoploid Triploid Tetraploid Euploidy is an excess or deficiency for the entire complement of chromosomes. Euploidy Organisms w Plants, amphibians, lower reptiles w Some cells in other organisms (liver, midgut, etc.) Root Causes w Nondisjunction w Autoreduplication Euploidy (continued) Origins w Autopolyploidy { Often involving individuals within the same species. { Infertile. Propagation by cloning. w Allopolyploidy { Often involving individuals from different species. { Fertile. Dosage Concerns Examples Numerical Chromosome Mutations Aneuploidy Excess or Defficiency in a single chromosome. 1. Monosomy 2. Disomy (only if normally cell is not diploid) 3. Trisomy 4. Tetrasomy Aneuploidy Chromosomes shown at metaphase Normal Diploid Aneuploid Monosomic Trisomic Tetrasomic Aneuploidy is an excess or deficiency for fewer than the entire complement of chromosomes. Aneuploidy Organisms w All organisms w Variability in how they deal with it Root Causes w Nondisjunction w Results of Chromosome Structure Mutations Aneuploidy -- Examples Plants w Jimson Weed (Datura stramonium) Animals w Drosophila: Triploid females are viable & fertile. Trisomy flies are not! w Human (autosomal): { Trisomy 13 (Patau Syndrome) 1:20,000 { Trisomy 18 (Edwards Syndrome) 1:10,000 { Trisomy 21 (Down Syndrome) 1:700 { Other rare ones (7, 9, 12, 14, & 22) Aneuploidy Examples w Human Sex Determining Chromosomes 1. -

Chromosomal Disorders
Understanding Genetic Tests and How They Are Used David Flannery,MD Medical Director American College of Medical Genetics and Genomics Starting Points • Genes are made of DNA and are carried on chromosomes • Genetic disorders are the result of alteration of genetic material • These changes may or may not be inherited Objectives • To explain what variety of genetic tests are now available • What these tests entail • What the different tests can detect • How to decide which test(s) is appropriate for a given clinical situation Types of Genetic Tests . Cytogenetic . (Chromosomes) . DNA . Metabolic . (Biochemical) Chromosome Test (Karyotype) How a Chromosome test is Performed Medicaldictionary.com Use of Karyotype http://medgen.genetics.utah.e du/photographs/diseases/high /peri001.jpg Karyotype Detects Various Chromosome Abnormalities • Aneuploidy- to many or to few chromosomes – Trisomy, Monosomy, etc. • Deletions – missing part of a chromosome – Partial monosomy • Duplications – extra parts of chromosomes – Partial trisomy • Translocations – Balanced or unbalanced Karyotyping has its Limits • Many deletions or duplications that are clinically significant are not visible on high-resolution karyotyping • These are called “microdeletions” or “microduplications” Microdeletions or microduplications are detected by FISH test • Fluorescence In situ Hybridization FISH fluorescent in situ hybridization: (FISH) A technique used to identify the presence of specific chromosomes or chromosomal regions through hybridization (attachment) of fluorescently-labeled DNA probes to denatured chromosomal DNA. Step 1. Preparation of probe. A probe is a fluorescently-labeled segment of DNA comlementary to a chromosomal region of interest. Step 2. Hybridization. Denatured chromosomes fixed on a microscope slide are exposed to the fluorescently-labeled probe. Hybridization (attachment) occurs between the probe and complementary (i.e., matching) chromosomal DNA. -

NIPT Fact Sheet
Victorian Clinical Genetics Services Murdoch Childrens Research Institute The Royal Children's Hospital Flemington Road, Parkville VIC 3052 P (03) 8341 6201 W vcgs.org.au NIPT fact sheet High risk for Monosomy X This fact sheet is for women and their partners who receive a high risk Possible explanations for this result for monosomy X from the perceptTM non-invasive prenatal test (NIPT) offered by the Victorian Clinical Genetics Services (VCGS). This high risk result: information may not be applicable to test results reported by other NIPT There is a chance that the baby has monosomy service providers. X however, only about 1 in 5 (or 20%) high risk As part of the service offered by VCGS, women and their partners have monosomy X results are associated with a true the opportunity to discuss their result with a genetic counsellor at no finding of monosomy X in the baby. additional cost. Genetic counsellors are experts in assisting people to The only way to provide a definitive diagnosis understand genetic test results and make informed decisions about is to have a diagnostic procedure (CVS or further testing options. amniocentesis) with chromosome testing. High risk results for monosomy X are often ‘false positive’ test results. A false positive result What does this result mean? means that although NIPT indicates a high risk NIPT is a way for women to get an accurate estimate of the chance of monosomy X, the baby does not have this that their baby has one of the most common chromosome conditions: condition. trisomy 21 (Down syndrome), trisomy 18 and trisomy 13. -

Chromosomal Study in Newborn Infants with Congenital Anomalies in Assiut University Hospital: Cross-Sectional Study
The Egyptian Journal of Medical Human Genetics (2011) 12, 79–90 Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com ORIGINAL ARTICLE Chromosomal study in newborn infants with congenital anomalies in Assiut University hospital: Cross-sectional study Yasir A. Mohammed a,*, Rabah M. Shawky b, Amal A.S. Soliman c, Maher M. Ahmed c a Tema Hospital, Ministry of Health, Sohag, Egypt b Pediatric Department, Ain Shams University, Cairo, Egypt c Pediatric Department, Assiut University, Assiut, Egypt Received 8 August 2010; accepted 8 January 2011 KEYWORDS Abstract In 40–60% of congenital malformations, the cause is unknown. Genetic factors account Chromosomes; for approximately 15%; environmental factors produce approximately 10%; a combination of Congenital anomalies; genetic and environmental influences produces 20–25%. The study aims to document prevalence Karyotype; and patterns of congenital malformations detected at birth in Assiut University hospital and clarify Malformations; underlying chromosomal abnormalities of such malformations. Also possible predisposing factors Egypt will be studied. Newborns with apparent congenital anomalies were selected from 5000 newborn infants delivered consecutively at the department of Obstetrics and Gynecology within 7 months. Full maternal his- tory, family history, perinatal history, pedigree construction as well as clinical examinations and investigations including karyotype were done. Congenital anomalies were found in 103 cases with a prevalence of 2.06% with male to female ratio of 1.7:1. Skeletal system anomalies had the highest * Corresponding author. Address: Sohag Governorate, Al Gomhoria Street, Egypt. Tel.: +022 0105339867/93789863. E-mail addresses: [email protected] (Y.A. Mohammed), shawkyr- [email protected] (R.M. -

Hyperkinetic Movement Disorders Differential Diagnosis and Treatment
Hyperkinetic Movement Disorders Differential diagnosis and treatment Albanese_ffirs.indd i 1/23/2012 10:47:45 AM Wiley Desktop Edition This book gives you free access to a Wiley Desktop Edition – a digital, interactive version of your book available on your PC, Mac, laptop or Apple mobile device. To access your Wiley Desktop Edition: • Find the redemption code on the inside front cover of this book and carefully scratch away the top coating of the label. • Visit “http://www.vitalsource.com/software/bookshelf/downloads” to download the Bookshelf application. • Open the Bookshelf application on your computer and register for an account. • Follow the registration process and enter your redemption code to download your digital book. • For full access instructions, visit “http://www.wiley.com/go/albanese/movement” Companion Web Site A companion site with all the videos cited in this book can be found at: www.wiley.com/go/albanese/movement Albanese_ffirs.indd ii 1/23/2012 10:47:45 AM Hyperkinetic Movement Disorders Differential diagnosis and treatment EDITED BY Alberto Albanese MD Professor of Neurology Fondazione IRCCS Istituto Neurologico Carlo Besta Università Cattolica del Sacro Cuore, Milan, Italy Joseph Jankovic MD Professor of Neurology Director, Parkinson’s Disease Center and Movement Disorders Clinic Department of Neurology Baylor College of Medicine Houston, TX, USA A John Wiley & Sons, Ltd., Publication Albanese_ffirs.indd iii 1/23/2012 10:47:45 AM This edition first published 2012, © 2012 by Blackwell Publishing Ltd Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing program has been merged with Wiley’s global Scientific, Technical and Medical business to form Wiley-Blackwell.