Bacterial Endophyte Communities of Three Agricultural Important Grass
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microbial Diversity of Molasses Containing Tobacco (Maassel) Unveils Contamination with Many Human Pathogens
European Review for Medical and Pharmacological Sciences 2021; 25: 4919-4929 Microbial diversity of molasses containing tobacco (Maassel) unveils contamination with many human pathogens M.A.A. ALQUMBER Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Albaha University, Saudi Arabia Abstract. – OBJECTIVE: Tobacco smoking drugs in today’s modern world. Different meth- remains a worldwide health issue, and the use of ods are currently used to consume tobacco, in- flavored varieties (maassel) embedded in glyc- cluding cigarettes, cigars and waterpipes1. Water- erine, molasses, and fruit essence via shisha pipe (shisha) smoking continues to rise globally2. paraphernalia (waterpipe) is growing globally. Smoking flavored tobacco (maassel), through the 16S rRNA gene pyrosequencing was conduct- shisha, is becoming a global preventable cause of ed on 18 different varieties representing 16 fla- 3,4 vors and three brands in order to study the mi- morbidity and mortality . crobiota of maassel and find whether it contains Scientists studied the chemical composition of pathogenic bacteria. tobacco for many years and illustrated the total MATERIALS AND METHODS: The samples number of chemicals identified in tobacco during were selected randomly from the most utilized the years from 1954 to 20055. In addition, a com- brands within Albaha, Saudi Arabia as deter- prehensive review of these chemicals’ classifica- mined through a questionnaire of 253 smok- ers. In addition, ten-fold serially diluted sam- tion, concentration and changes with time due ples were inoculated on blood agar, MacConkey to changes in the shape, design and composition agar, half-strength trypticase soy agar and malt of cigarettes was reported almost a decade ago6. -

Table S1. Bacterial Otus from 16S Rrna
Table S1. Bacterial OTUs from 16S rRNA sequencing analysis including only taxa which were identified to genus level (those OTUs identified as Ambiguous taxa, uncultured bacteria or without genus-level identifications were omitted). OTUs with only a single representative across all samples were also omitted. Taxa are listed from most to least abundant. Pitcher Plant Sample Class Order Family Genus CB1p1 CB1p2 CB1p3 CB1p4 CB5p234 Sp3p2 Sp3p4 Sp3p5 Sp5p23 Sp9p234 sum Gammaproteobacteria Legionellales Coxiellaceae Rickettsiella 1 2 0 1 2 3 60194 497 1038 2 61740 Alphaproteobacteria Rhodospirillales Rhodospirillaceae Azospirillum 686 527 10513 485 11 3 2 7 16494 8201 36929 Sphingobacteriia Sphingobacteriales Sphingobacteriaceae Pedobacter 455 302 873 103 16 19242 279 55 760 1077 23162 Betaproteobacteria Burkholderiales Oxalobacteraceae Duganella 9060 5734 2660 40 1357 280 117 29 129 35 19441 Gammaproteobacteria Pseudomonadales Pseudomonadaceae Pseudomonas 3336 1991 3475 1309 2819 233 1335 1666 3046 218 19428 Betaproteobacteria Burkholderiales Burkholderiaceae Paraburkholderia 0 1 0 1 16051 98 41 140 23 17 16372 Sphingobacteriia Sphingobacteriales Sphingobacteriaceae Mucilaginibacter 77 39 3123 20 2006 324 982 5764 408 21 12764 Gammaproteobacteria Pseudomonadales Moraxellaceae Alkanindiges 9 10 14 7 9632 6 79 518 1183 65 11523 Betaproteobacteria Neisseriales Neisseriaceae Aquitalea 0 0 0 0 1 1577 5715 1471 2141 177 11082 Flavobacteriia Flavobacteriales Flavobacteriaceae Flavobacterium 324 219 8432 533 24 123 7 15 111 324 10112 Alphaproteobacteria -

Resuscitation of Soil Microbiota After > 70-Years of Desiccation
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.06.371641; this version posted November 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Resuscitation of soil microbiota after > 70-years of desiccation 2 Jun Zhao1‡, Dongfeng Chen1‡*, Wei Gao1,2, Zhiying Guo1,2, Zhongjun Jia1, 3 Marcela Hernández3* 4 1State Key Laboratory of Soil and Sustainable Agriculture. Institute of Soil Science, 5 Chinese Academy of Sciences. Nanjing, 210008, Jiangsu Province, China 6 2University of Chinese Academy of Sciences, Beijing 100049, China 7 3School of Environmental Sciences, Norwich Research Park, University of East 8 Anglia, Norwich, UK 9 10 ‡These authors contribute equally to this work 11 12 * Correspondence: 13 Dongfeng Chen: [email protected] 14 Marcela Hernández: [email protected] 15 Running title: Soil microbes after long-term desiccation 16 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.06.371641; this version posted November 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 17 Abstract 18 The abundance and diversity of bacteria in 24 historical soil samples under air- 19 dried storage conditions for more than 70 years were assessed by quantification and 20 high-throughput sequencing analysis of 16S rRNA genes. -

Actinomycetes Isolated from Wetland and Hill Paddy During the Warm and Cool Seasons in Sarawak, East Malaysia
ACTINOMYCETES ISOLATED FROM WETLAND AND HILL PADDY DURING THE WARM AND COOL SEASONS IN SARAWAK, EAST MALAYSIA Ann Anni Basik*, Holed Juboi, Sunita Sara Gill Shamsul, Jean-Jacques Sanglier and Tiong Chia Yeo Address(es): Ann Anni Basik 1 Sarawak Biodiversity Centre, Km. 20 Jalan Borneo Heights, Semengoh, 93250 Kuching, Sarawak, Malaysia. *Corresponding author: [email protected] doi: 10.15414/jmbfs.2020.9.4.774-780 ARTICLE INFO ABSTRACT Received 12. 3. 2018 As part of the Natural Product Discovery programme at Sarawak Biodiversity Centre (SBC), our study targeted isolation and evaluation Revised 4. 9. 2019 of actinomycetes diversity from paddy rice fields. Samples from two types of paddy farming system practiced in Sarawak, wet land and Accepted 11. 9. 2019 hill paddy, were collected and processed leading to the selection of 578 strains distributed among 24 genera and 10 families. Analysis Published 3. 2. 2020 using phylogenetic clustering indicated a total of 159 taxonomic units (TU). The taxonomic position and the ranking of the TU allowed their classification in 4 novel species, 61 putative novel species and 94 known species or species of uncertain position. The high genus diversity and percentage of novel or putative novel species demonstrate the biodiversity potential of Sarawak ecosystems, even in man- Regular article managed ecosystems. Keywords: paddy field, actinomycetes, ranking, taxonomic unit INTRODUCTION sequence identity (Gevers et al., 2005). However, species can be differentiated at a level of 98.2 – 99 % 16S rRNA similarity (Kim et al., 2014). Isolation of rare actinomycetes from paddy rice (Oryza sativa L.) field in the Apart from the commonly collected soil samples, rhizospheric soil and roots were Kuching Division, Sarawak were made to evaluate their diversity and also included for the isolation of actinomycetes in this project. -

Impact of Cropping Systems, Soil Inoculum, and Plant Species Identity on Soil Bacterial Community Structure
Impact of Cropping Systems, Soil Inoculum, and Plant Species Identity on Soil Bacterial Community Structure Authors: Suzanne L. Ishaq, Stephen P. Johnson, Zach J. Miller, Erik A. Lehnhoff, Sarah Olivo, Carl J. Yeoman, and Fabian D. Menalled The final publication is available at Springer via http://dx.doi.org/10.1007/s00248-016-0861-2. Ishaq, Suzanne L. , Stephen P. Johnson, Zach J. Miller, Erik A. Lehnhoff, Sarah Olivo, Carl J. Yeoman, and Fabian D. Menalled. "Impact of Cropping Systems, Soil Inoculum, and Plant Species Identity on Soil Bacterial Community Structure." Microbial Ecology 73, no. 2 (February 2017): 417-434. DOI: 10.1007/s00248-016-0861-2. Made available through Montana State University’s ScholarWorks scholarworks.montana.edu Impact of Cropping Systems, Soil Inoculum, and Plant Species Identity on Soil Bacterial Community Structure 1,2 & 2 & 3 & 4 & Suzanne L. Ishaq Stephen P. Johnson Zach J. Miller Erik A. Lehnhoff 1 1 2 Sarah Olivo & Carl J. Yeoman & Fabian D. Menalled 1 Department of Animal and Range Sciences, Montana State University, P.O. Box 172900, Bozeman, MT 59717, USA 2 Department of Land Resources and Environmental Sciences, Montana State University, P.O. Box 173120, Bozeman, MT 59717, USA 3 Western Agriculture Research Center, Montana State University, Bozeman, MT, USA 4 Department of Entomology, Plant Pathology and Weed Science, New Mexico State University, Las Cruces, NM, USA Abstract Farming practices affect the soil microbial commu- then individual farm. Living inoculum-treated soil had greater nity, which in turn impacts crop growth and crop-weed inter- species richness and was more diverse than sterile inoculum- actions. -

Metagenomic Characterization Reveals Pronounced Seasonality in the Diversity and Structure of the Phyllosphere Bacterial Community in a Mediterranean Ecosystem
microorganisms Article Metagenomic Characterization Reveals Pronounced Seasonality in the Diversity and Structure of the Phyllosphere Bacterial Community in a Mediterranean Ecosystem Despoina Vokou 1,*, Savvas Genitsaris 2 , Katerina Karamanoli 3, Katerina Vareli 4, Marina Zachari 4, Despoina Voggoli 4, Nikolaos Monokrousos 5, John Maxwell Halley 4 and Ioannis Sainis 4 1 Department of Ecology, School of Biology, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece 2 School of Economics, Business Administration and Legal Studies, International Hellenic University, 57001 Thermi, Greece; [email protected] 3 School of Agriculture, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece; [email protected] 4 Department of Biological Applications and Technology, University of Ioannina, 45110 Ioannina, Greece; [email protected] (K.V.); [email protected] (M.Z.); [email protected] (D.V.); [email protected] (J.M.H.); [email protected] (I.S.) 5 Department of Soil Science of Athens, Hellenic Agricultural Organization-Demeter, Institute of Soil and Water Resources, 14123 Lykovrisi, Greece; [email protected] * Correspondence: [email protected] Received: 17 September 2019; Accepted: 29 October 2019; Published: 1 November 2019 Abstract: We explore how the phyllosphere microbial community responds to a very seasonal environment such as the Mediterranean. For this, we studied the epiphytic bacterial community of a Mediterranean ecosystem in summer and winter, expecting to detect seasonal differences at their maximum. With high-throughput sequencing (HTS), we detected the operational taxonomic units (OTUs) present in the phyllosphere and also in the surrounding air. The epiphytic community is approximately five orders of magnitude denser than the airborne one and is made almost exclusively by habitat specialists. -

Shifts in Soil Bacterial Communities Associated with the Potato
Shifts in soil bacterial communities associated with the potato rhizosphere in response to aromatic sulfonate amendments Caroline Sablayrolles, Jan Dirk van Elsas, Joana Falcão Salles To cite this version: Caroline Sablayrolles, Jan Dirk van Elsas, Joana Falcão Salles. Shifts in soil bacterial communities associated with the potato rhizosphere in response to aromatic sulfonate amendments. Applied Soil Ecology, Elsevier, 2013, 63, pp.78-87. 10.1016/j.apsoil.2012.09.004. hal-02147674 HAL Id: hal-02147674 https://hal.archives-ouvertes.fr/hal-02147674 Submitted on 4 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/23717 Official URL: https://doi.org/10.1016/j.apsoil.2012.09.004 To cite this version: İnceoğlu, Özgül and Sablayrolles, Caroline and van Elsas, Jan Dirk and Falcão Salles, Joana Shifts in soil bacterial communities associated with the potato rhizosphere in response to aromatic sulfonate amendments. (2013) Applied Soil Ecology, 63. 78-87. -

Inter-Domain Horizontal Gene Transfer of Nickel-Binding Superoxide Dismutase 2 Kevin M
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.12.426412; this version posted January 13, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Inter-domain Horizontal Gene Transfer of Nickel-binding Superoxide Dismutase 2 Kevin M. Sutherland1,*, Lewis M. Ward1, Chloé-Rose Colombero1, David T. Johnston1 3 4 1Department of Earth and Planetary Science, Harvard University, Cambridge, MA 02138 5 *Correspondence to KMS: [email protected] 6 7 Abstract 8 The ability of aerobic microorganisms to regulate internal and external concentrations of the 9 reactive oxygen species (ROS) superoxide directly influences the health and viability of cells. 10 Superoxide dismutases (SODs) are the primary regulatory enzymes that are used by 11 microorganisms to degrade superoxide. SOD is not one, but three separate, non-homologous 12 enzymes that perform the same function. Thus, the evolutionary history of genes encoding for 13 different SOD enzymes is one of convergent evolution, which reflects environmental selection 14 brought about by an oxygenated atmosphere, changes in metal availability, and opportunistic 15 horizontal gene transfer (HGT). In this study we examine the phylogenetic history of the protein 16 sequence encoding for the nickel-binding metalloform of the SOD enzyme (SodN). A comparison 17 of organismal and SodN protein phylogenetic trees reveals several instances of HGT, including 18 multiple inter-domain transfers of the sodN gene from the bacterial domain to the archaeal domain. -

Impact of Plant Species, N Fertilization and Ecosystem Engineers on the Structure and Function of Soil Microbial Communities
IMPACT OF PLANT SPECIES, N FERTILIZATION AND ECOSYSTEM ENGINEERS ON THE STRUCTURE AND FUNCTION OF SOIL MICROBIAL COMMUNITIES Dissertation zur Erlangung des mathematisch-naturwissenschaftlichen Doktorgrades "Doctor rerum naturalium" der Georg-August-Universität Göttingen im Promotionsprogramm Biologie der Georg-August University School of Science (GAUSS) vorgelegt von Birgit Pfeiffer aus Forst/ Lausitz Göttingen 2013 Betreuungsausschuss Prof. Dr. Rolf Daniel, Genomische und angewandte Mikrobiologie, Institut für Mikrobiologie und Genetik; Georg-August-Universität Göttingen PD Dr. Michael Hoppert, Allgemeine Mikrobiologie, Institut für Mikrobiologie und Genetik; Georg-August-Universität Göttingen Mitglieder der Prüfungskommission Referent/in: Prof. Dr. Rolf Daniel, Genomische und angewandte Mikrobiologie, Institut für Mikrobiologie und Genetik; Georg-August-Universität Göttingen Korreferent/in: PD Dr. Michael Hoppert, Allgemeine Mikrobiologie, Institut für Mikrobiologie und Genetik; Georg-August-Universität Göttingen Weitere Mitglieder der Prüfungskommission: Prof. Dr. Hermann F. Jungkunst, Geoökologie / Physische Geographie, Institut für Umweltwissenschaften, Universität Koblenz-Landau Prof. Dr. Stefanie Pöggeler, Genetik eukaryotischer Mikroorganismen, Institut für Mikrobiologie und Genetik, Georg-August-Universität Göttingen Prof. Dr. Stefan Irniger, Molekulare Mikrobiologie und Genetik, Institut für Mikrobiologie und Genetik, Georg-August-Universität Göttingen Jun.-Prof. Dr. Kai Heimel, Molekulare Mikrobiologie und Genetik, Institut für Mikrobiologie und Genetik, Georg-August-Universität Göttingen Tag der mündlichen Prüfung: 20.12.2013 Two things are necessary for our work: unresting patience and the willingness to abandon something in which a lot of time and effort has been put. Albert Einstein, (Free translation from German to English) Dedicated to my family. Table of contents Table of contents Table of contents I List of publications III A. GENERAL INTRODUCTION 1 1. BIODIVERSITY AND ECOSYSTEM FUNCTIONING AS IMPORTANT GLOBAL ISSUES 1 2. -

Bacillus Mangrovi Sp. Nov., Isolated from a Sediment Sample of Coringa Mangrove Forest, India
Author version : International Journal of Systematic and Evolutionary Microbiology,vol.67(7);2017; 2219-2224 Bacillus mangrovi sp. nov., isolated from a sediment sample of Coringa mangrove forest, India Vasundhera Gupta1, Pradip Kumar Singh1, Suresh Korpole1, Naga Radha Srinivas Tanuku2, Anil Kumar Pinnaka.*1 1MTCC-Microbial Type Culture Collection & Gene Bank, CSIR-Institute of Microbial Technology, Chandigarh-160036, India 2CSIR-National Institute of Oceanography, Regional Centre, 176, Lawsons Bay Colony, Visakhapatnam-530017, India Address for correspondence *Dr. P. Anil Kumar MTCC-Microbial Type Culture Collection & Gene Bank, CSIR- Institute of Microbial Technology, Chandigarh-160036, India E-mail: [email protected] Telephone: +91-172-6665170 Running title: Bacillus mangrovi sp. nov. Subject category New taxa (Firmicutes) The GenBank/EMBL/DDBJ accession number for the 16S rRNA gene sequence of strain AK61T is HG974242. A facultatively anaerobic, endospore forming, alkali tolerant, Gram-positive-staining, motile, rod shaped bacterium, designated strain AK61T, was isolated from a sediment sample collected from Coringa mangrove forest, India. Colonies were circular, 1.5 mm in diameter, shiny, smooth, yellowish and convex with entire margin after 48 h growth at 30oC. Growth occurred at 15-42 oC, 0- 3% (w/v) NaCl and pH of 6-9. Strain AK61T was positive for amylase activity and negative for oxidase, catalase, aesculinase, caseinase, cellulase, DNase, gelatinase, lipase and urease activities. The fatty acids were dominated by branched with iso-, anteiso-, saturated fatty acids with a high abundance of iso-C14:0, iso-C15:0, anteiso-C15:0 and iso-C16:0; the cell-wall peptidoglycan contained - meso-diaminopimelic acid as the diagnostic diamino acid; and MK-7 is the major menaquinone. -

Comparison of Methods to Identify Pathogens and Associated Virulence Functional Genes in Biosolids from Two Different Wastewater Treatment Facilities in Canada
RESEARCH ARTICLE Comparison of Methods to Identify Pathogens and Associated Virulence Functional Genes in Biosolids from Two Different Wastewater Treatment Facilities in Canada Etienne Yergeau1*, Luke Masson2, Miria Elias1, Shurong Xiang3, Ewa Madey4, a11111 Hongsheng Huang5, Brian Brooks5, Lee A. Beaudette3 1 National Research Council Canada, Energy Mining and Environment, Montreal, Qc, Canada, 2 National Research Council Canada, Human Health Therapeutics, Montreal, Qc, Canada, 3 Environment Canada, Biological Assessment and Standardization Section, Ottawa, On, Canada, 4 Canadian Food Inspection Agency, Fertilizer Safety Office, Plant Health & Biosecurity Directorate, Ottawa, On, Canada, 5 Canadian Food Inspection Agency, Ottawa Laboratory – Fallowfield, Ottawa, On, Canada OPEN ACCESS * [email protected] Citation: Yergeau E, Masson L, Elias M, Xiang S, Madey E, Huang H, et al. (2016) Comparison of Methods to Identify Pathogens and Associated Abstract Virulence Functional Genes in Biosolids from Two Different Wastewater Treatment Facilities in Canada. The use of treated municipal wastewater residues (biosolids) as fertilizers is an attractive, PLoS ONE 11(4): e0153554. doi:10.1371/journal. inexpensive option for growers and farmers. Various regulatory bodies typically employ pone.0153554 indicator organisms (fecal coliforms, E. coli and Salmonella) to assess the adequacy and Editor: Leonard Simon van Overbeek, Wageningen efficiency of the wastewater treatment process in reducing pathogen loads in the final University and Research Centre, NETHERLANDS product. Molecular detection approaches can offer some advantages over culture-based Received: October 28, 2015 methods as they can simultaneously detect a wider microbial species range, including Accepted: March 31, 2016 non-cultivable microorganisms. However, they cannot directly assess the viability of the Published: April 18, 2016 pathogens. -
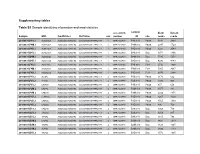
Supplementary Tables Table S1 Sample Identifying Information And
Supplementary tables Table S1 Sample identifying information and read statistics accession sample #raw #clean Sample MID FwdPrimer RvPrimer run number ID site reads reads 20100831.P4S.1 ACACGAGA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 P4S.910 Palsa 3831 2893 20100831.P4M.1 ACATAGCA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 P4M.910 Palsa 2047 1729 20100831.P4D.1 ACGATACA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 P4D.910 Palsa 3221 2543 20100831.B4S.1 ACTGCTCA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 B4S.910 Bog 1971 1366 20100831.B4M.1 AGCAGCGC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 B4M.910 Bog 5530 5251 20100831.B4D.1 AGCGTGCA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 B4D.910 Bog 4296 4143 20100831.F4S.1 AGTCTAGC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 F4S.910 Fen* 2716 1890 20100831.F4M.1 ATGACTGA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 F4M.910 Fen* 5005 3987 20100831.F4D.1 ATGCACGC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 F4D.910 Fen* 2870 2384 20100901.P1S.2 ACTGAT ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P1S.910 Palsa 1170 662 20100901.P2S.2 ATGTGT ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P2S.910 Palsa 1320 923 20100901.P3S.2 CACAGT ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P3S.910 Palsa 857 656 20100901.P2M.2 CACTAC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P2M.910 Palsa 1077 881 20100901.P3M.2 CAGCAT ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P3M.910 Palsa 2032 1474 20100901.P1D.2 CTAGAGC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT