Effect of Temperature on Saccharification And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prema Shanti Is Our Original Word, Combined Two Sanskrit Words, Means Prema Shanti "Heaven-Sent Love and Inner Peace"
IN ORIG dƌĂĚŝƟŽŶĂůĂƌůĞLJDŝƐŽdƌd ĂĚĚŝƟƟŽŶĂů ĂƌƌůĞLJ DDŝƐŽŽ dƌĂĚŝƟŽŶĂůZŝĐĞDŝƐŽdƌĂĚĚŝƟƟŽŶĂůů ZŝŝĐĞ DŝŝƐŽŽ EŽƚŽEĂƚƵƌĂů^ĞĂƐĂůƚEŽE ƚŽƚŽ EĂƚƚƵƌĂů ^ĞĂ ƐĂůůƚ Old and Knowing th to the wing the e New journey Revie ~ ~KƌŐĂŶŝĐKKƌŐĂĂŶŝŝĐ~ ~KƌŐĂŶŝĐKKƌŐĂĂŶŝŝĐ~ Prema Shanti is our original word, combined two Sanskrit words, means Prema Shanti "heaven-sent love and inner peace". プレマシャンティ Ⓡ Freshly Ɖacked͕ nonͲboiled. erƟĮed :^ Freshly Ɖacked͕ nonͲboiled. erƟĮed :^ From Noto Weninsula ƋuasiͲnaƟonal Wark. hnreĮned͕ dŚĞĂƵƚŚĞŶƟĐ:ĂƉĂŶĞƐĞƚƌĂĚŝƟŽŶĂůĨŽŽĚƐĨŽƌĨƵƚƵƌĞŐĞŶĞƌĂƟŽŶ Organic. >ighter Ňaǀor miso made of Organic. FullͲbodied and matured deeƉ Ňaǀor MineralͲrich͕ contains more than ϵϬ tyƉes of minerals in organically grown whole soybeans and barley miso made of organically grown whole sea water mineral balance. No chemicals added͕ DƵĐŚůŝŬĞƚƌĂĚŝƟŽŶĂů:ĂƉĂŶĞƐĞĐƵŝƐŝŶĞ͕:ĂƉĂŶĞƐĞƵƐĞĚƚŽůŝǀĞŽŶĞĐŽůŽŐŝĐĂůŽƌĚĞƌĂŶĚƐĞĂƐŽŶĂůŝƚLJ͘dŚĂŶŬƐĨŽƌ with Η<oũiΗ Miso starter͕barley malt͕ house soybeans and rice with Η<oũiΗ Miso starter͕ rice naturally moist. Made of beauƟful emerald green ĨĂƌŵĞƌƐ ĂŶĚ ĂƌƟƐĂŶƐ͕ WƌĞŵĂ ƐŚĂŶƟ ŝŶƚƌŽĚƵĐĞƐ ƚƌĂĚŝƟŽŶĂůůLJ ĂŶĚ ĂƵƚŚĞŶƟĐĂůůLJ ŵĂĚĞ ŝŶŐƌĞĚŝĞŶƚƐ ƚŽ ĐĂƌƌLJ ŽŶ deǀeloƉed with naturalͲhabitat yeasts͕ aged in malt͕ house deǀeloƉed with naturalͲhabitat NotoͲsea water͕ ƉuriĮed͕ reduced͕ and concentrated. ΗďĞĂƵƚLJŽĨƚƌĂĚŝƟŽŶĂů:ĂƉĂŶĞƐĞĨŽŽĚƐΗĂŶĚΗŐƌĂĐĞĨƵůŶĞƐƐŽĨ:ĂƉĂŶĞƐĞĨŽƵƌƐĞĂƐŽŶƐΗ͘ cedar kegs more than a year in the tradiƟonal yeasts͕ aged in cedar kegs more than a year in Follow and reƉlicate tradiƟonal salt making with indirectly methods carried by Marukawa family*1 for the tradiƟonal methods carried by Marukawa boiled with low temƉerature with the doubleͲboil Θ low 1ϬϬyers. n allͲƉurƉose miso͕ great for eǀery family*1 for 1ϬϬyers. n allͲƉurƉose miso͕ great temƉerate crystallinjaƟon method methods;Ɖatent day use. for winter Ɵme. ƉrotectedͿ. Ňaǀorful and gentle͕ smooth͕ mellow Ňaǀor. Criteria for Prema Shanti Selection Enhance natural Ňaǀor of any kind of dishes. 345g(pouch) - 6/case item:00100008 345g(pouch) - 6/case item:00100005 100g - 100/case item:00100015 1. -

20180910 Matcha ENG.Pdf
Matcha served with wagashi Sencha served with wagashi Whole tea leaves ground into fine, ceremonial grade Choose from three excellent local teas curated by powder and whipped into a foamy broth using a our expert staff. Select one Kagoshima wagashi to method unchanged for over 400 years. Choose one accompany your tea for a refreshing break. Kagoshima wagashi to accompany your drink for the perfect pairing of bitter and sweet. ¥1,000 ¥850 Please choose your tea and wagashi from the following pages. All prices include tax. Please choose your sencha from the options below. Chiran-cha Tea from fields surrounding the samurai town of Chiran. Slightly sweet with a clean finish. Onejime-cha Onejime produces the earliest first flush tea in Kagoshima. Sharp, clean flavour followed by a gently lingering bitterness. Kirishima Organic Matcha The finest organically cultivated local matcha from the mountains of Kirishima. Full natural flavour with a slightly astringent edge. Oku Kirishima-cha Tea from the foothills of the Kirishima mountain range. Balanced flavour with refined umami and an astringent edge. Please choose your wagashi from the options below. Seasonal Namagashi(+¥200) Akumaki Sweets for the tea ceremony inspired by each season. Glutinous rice served with sweet toasted soybean Ideal with a refreshing cup of matcha. flour. This famous snack was taken into battle by tough Allergy information: contains yam Satsuma samurai. Allergy information: contains soy Karukan Hiryu-zu Local yams combined with refined rice flour and white Sweet bean paste, egg yolk, ginkgo nuts, and shiitake sugar to create a moist steamed cake. Created 160 mushrooms are combined in this baked treat that has years ago under the orders of Lord Shimadzu Nariakira. -

Vegan Lunch Box : 150 Amazing, Animal-Free Lunches Kids and Grown-Ups Will Love! / Jennifer Mccann
1600940729 text_rev.qxd 4/21/08 8:53 AM Page i PRAISE FOR VEGAN LUNCH BOX “Jennifer McCann’s cookbook makes vegan cooking accessible and fun. It’s informative but not stuffy, detailed yet concise, and the recipes are creative without being difficult. There are so many delicious, well put to- gether options here, it’s not only perfect for kids but for anyone who ever eats lunch!” —Isa Chandra Moskowitz, author of VEGANOMICON “Being a vegan kid just got a lot easier! The menus in Vegan LunchLunch BoxBox make it easy to plan a balanced and nutritious lunch for your kids (or your- self!). The variety alone makes it worth having.” —Erin Pavlina, author of RAISING VEGAN CHILDREN IN A NON-VEGAN WORLD “Destined to become a classic, this is the book vegan parents have been waiting for. And who knew? A vegan mom started a blog describing the lunches she made for her son for one school year, and it won the 2006 Bloggie Award for “Best Food Blog” (NOT “best VEGETARIAN food blog,” but “Best Food Blog,” period!). It inspired, delighted, and motivated not only vegan parents, but omnivores bored with their own lackluster lunches. This book will continue delighting with recipes that are as innovative, kid- pleasing, and healthful as they are delicious.” —Bryanna Clark Grogan, author of NONNA’S ITALIAN KITCHEN 1600940729 text_rev.qxd 4/21/08 8:53 AM Page ii This page intentionally left blank 1600940729 text_rev.qxd 4/21/08 8:53 AM Page iii Vegan Lunch Box 1600940729 text_rev.qxd 4/21/08 8:53 AM Page iv This page intentionally left blank 1600940729 text_rev.qxd 4/21/08 8:53 AM Page v Vegan Lunch Box 150 Amazing, Animal-Free Lunches Kids and Grown-Ups Will Love! Jennifer McCann A Member of the Perseus Books Group 1600940729 text_rev.qxd 4/21/08 8:53 AM Page vi Many of the designations used by manufacturers and sellers to distinguish their products are claimed as trademarks. -

Kouji Cuisine Homemade Miso Amazake Soy Bean Dregs Miso
KOUJI CUISINE Restaurant Menu Restaurant Kouji THE CAN SHIO KOUJI MIRIN AMAZAKE MISO TOFU & SOYMILK SOY BEAN DREGS VEGETABLE & LEGUMES copyright © 2018 THE-CAN Co., Ltd. All rights reserved KOUJI CUISINE Handmade Brewed Creative Dishes Based with soybeans, fermented and brewed ingredients, followed with seasonal vegetables for variations. Our menu contains foods between light food and banquets. SHIO KOUJI MISO ESSENCE Shio Koji is made of Zhou Nan Sea Salt and organic rice The miso fermented using brown rice and black THE SHARING OF FOOD WITH also known as Japanese fermented ingredient and kernelled rice has higher nutritious value. It is contains more nutrients because of the action of healthy and delicious, and most suitable for use in OTHERS KEEPS PEOPLE CONNECTING. enzyme and microorganisms such as lactic acid bacteria cold dishes and as a dipping sauce. and yeast. Shio Koji can break down the starch and protein contained in the ingredients to bring out the With Our Pure And Natural Spices Giving The Rich Flavours, umami and sweetness to make the food delicious. Taste The Purest Flavor Of Food. HOMEMADE MISO SOY BEAN DREGS Kouji, organic rice and the domestic non-GMO Tofu dregs is a pulp consisting of insoluble parts of soybeans are sealed for the probiotics to generate the soybean that remains after pureed soybeans are flavors naturally without adding any chemical filtered in the production of soy milk and tofu, it’s substances. The naturally fermented substances relatively high in protein which makes it an excellent possess the activity of probiotics, so that you can choice for vegetarian diets. -

Food Menu 6.30 12.80
AYA M Food Menu AK EN IZ U Starter Edamame 揚げ漬枝豆 5.80 deep fried sweet soy marinated edamame beans VTsukemono 本日の丸ごとお野菜のお漬け物 6.30 house-made assorted Japanese style pickled vegetables VSpicy Agedashi Tofu スパイシー揚げ出し豆腐 6.30 deep fried tofu with chili sauce in dashi broth Salad VYasai 盛り盛り温野菜 8.80 steamed assorted vegetables with tofu & anchovy dipping sauce VTofu & Avocado Salad 豆腐アボカドサラダ 8.80 with guuu'd house dressing Ceviche Salad セビーチェサラダ 12.80 assorted sashimi & fresh tomato salsa on crispy pastry with guuu’d dressing & wasabi mayo Buddha Style Bang-Bang Salad 棒棒鶏サラダ 12.80 steamed chicken breast with quinoa, seasonal chunky veggies, sesame-peanut dressing, & mixed nuts V Vegetarian Friendly Food Menu AYA M AK EN IZ U Cold Wagyu Tataki 和牛タタキ 10.80 wagyu striploin beef tataki with ponzu sauce Salmon Tataki サーモンタタキ 10.50 seasoned seared salmon sashimi with ponzu sauce Tuna Tataki ツナタタキ 11.00 seared salted tuna sashimi with ponzu sauce Aburi Saba Mackerel 炙りしめ鯖 6.50 / small 12.50 / large seared pickled mackerel with ponzu sauce DeeFried / PanFried Guuu’d Poutine 石焼きカレープーティーン 12.80 Japanese beef curry poutine in hot stone bowl Takoyaki たこ焼き 6.80 deep fried octopus ball Honey Bacon Fries ハニーベーコンフライ 8.50 fries with honey bacon with chili ketchup & mayo Ebi-Mayo 海老マヨ 11.00 deep fried prawns with spicy mayonnaise (6pc) Koji Karaage 塩麹漬け若鶏のジューシー唐揚げ 6.80 / small 13.50 / large salt rice-malt marinated deep fried chicken thigh with garlic mayo, edamame sauce, black sesame sauce, & sweet soy sauce V Vegetarian Friendly AYA M Food Menu AK EN IZ U -

Stir Fried Rice, Double Boiled Vegetable and Ginger Soup
Breakfast Open from 7:00 until 10:30 Sandbank Caviar Breakfast A romantic start to the day. We will transfer you by private speedboat to our secluded island, with a personal waiter in attendance to set up your own piece of beach with umbrella, blanket and your choice of breakfast basket. (Please bear in mind that this excursion is subject to weather conditions and tide times.)Paul Roger Blanc de Blanc Champagne served with Kaviari oscietra caviar 50g, Kaviari Kristol caviar 50g, and Salmon eggs 50g Blinis, black bread, deviled eggs, house smoked salmon, fingerling potatoes, shallot, parsley,Two Hundred Ninety Dollars per person $290.00 pp Floating Breakfast Enjoy your breakfast in your private pool. $100.00 Set Breakfast Accompanied by tropical fruit, choice of freshly squeezed juice, and brewed coffee or Ceylon tea. Maldivian Heritage Enjoy a traditional Maldivianbreakfast, composed of a salad of smoked tuna,onion,coconut, chili, lime and sour coppee leaf. All ingredients are finely chopped and mixed and accompanied by a fried omelette, freshly bakedchapati flatbread and biskeemiyaa, a fried dumpling filled with cabbage, egg, onion and curry leaf. Best paired with sweet tea. $36.00 Middle Eastern Organic chicken eggs baked in spiced vegetable tomato sauce, grilled pita bread, chickpea, avocado, and feta salad, pickles, garden greens and herbs. $35.00 pp All Prices are quoted in USD and are subject to 10% service charge and 12% government taxes Chinese Rice congee with soy sauce, ginger, and green onion served with traditional accompaniments, youtiao (Chinese donut), dim sum dumpling of the day, stir fried garden greens, steamed roots, and chilled bean curd. -

Mana Kitchen
mana kitchen Probiotic ・ Organic ・ Heirloom ・the concept・ Mana Kitchen is an organic, probiotic, life-nourishing restaurant that offers Japanese-Indonesian fusion food. We use as much organic heirloom vegetables grown in our permaculture garden as possible, provide menu that is health- and eco-conscious, and serve homemade probiotic seasoning and food. You are what you eat. Food is medicine in itself. Our aim is to provide food that maximizes human potential and sustains lives for generations. This is important because so much of the food that we consume nowadays is essentially life-less and nutrition-less. White rice grains won’t sprout if you plant them. The majority of rice consumed in Indonesia is green-revolution rice, which yields large volumes but dilutes the nutrition content. White wheat is similarly devoid of regenerative properties. Most vegetables sold at supermarkets are made from F1 seeds that are designed for one-generation consumption. Mana Kitchen provides food the way the earth blessed us with. We call it “awakening food” because when you consume food the most natural way, it awakens your soul, mind, body and all your senses. Of course, we do not allow GMO vegetables, MSG seasoning, microwaves, and other evils to enter our kitchen. *We are not a vegetarian restaurant but we decided against serving beef because it's the most unecological thing to produce. The popular red meat requires 28 times more land to produce than pork or chicken and 11 times more water. Production of beef meat accounts for a staggering 80 percent of the total greenhouse gas emitted by all livestock. -

'CRUMPET' Sesame, Pickled Kumquat Dipping Sauce
NIBBLES — WELSH LOBSTER ‘CRUMPET’ CORN RIBS, BREAD & LIME MISO, sesame, pickled kumquat dipping sauce — pumpkin seed dukkah 9.5 7.5 SMALL PLATES — VEGETABLE BURRATA, charred apricots, ancho chilli and orange oil 14.5 COFFEE ROASTED BEETROOT, basil, grapefruit amazake (Vg) 12.5 TEMPURA STEMS & HERBS, Szechuan salt, watermelon vinegar (V) 10.5 COURGETTE BORANI, goat’s yogurt, smoked harissa oil, malawah (V) 13.5 SWEET AND SOUR CAJUN PEPPERS, pickled jalapeno, corn bread crumb 13.50 CUCUMBER, black sesame sauce, Aleppo peanuts, lime & herbs (Vg) 12 CELERIAC SHAWARMA, bkeila, fermented tomato (V) 16.5 FIS H GRILLED OCTOPUS, borlotti bean piyaz, preserved lemon, sumac tropea onion 19.5 BBQ’D MACKEREL, vadouvan butter, pickles, samphire 16.5 STEAMED BLUE SHELL MUSSELS, barley shio, Namayasai herbs & greens 15 MEAT BEEF CARPACCIO (grass fed), beetroot, blackberry, Crowdie cheese 14.5 BEEF & SMOKED CHERRIES KEBABS, green chilli shatta, labneh 14.5 MAIN PLATES — JERUSALEM MIXED GRILL (mushroom (Vg) or chicken) Baharat onions, pickles, pita, tahini (Vg) 22/24 SHIITAKE BROWN RICE CONGEE, piatonni beans, almond XO sauce, fermented mouli (Vg) 19.50 WHOLE LEMON SOLE, spiced tomatoes, elderflower capers, baby gem 29 SADDLEBACK PORK CHOPS, charred peppers, pistachio & lime salsa 24.5 LAKE DISTRICT 45 DAY AGED RIBEYE, Café de ‘ROVI’ sauce, horseradish onions 36 S IDES — MIXED FINE BEANS, tomato, basil, crispy garlic (Vg) 7 OX HEART TOMATO, peach, and charred lemon salad (Vg) 7.5 EINKORN PITA, tahini (Vg) 4.5 Please do let your waiter know if you have any food allergies . A discretionary 12.5% service charge is added to all bills - this is shared by everyone here at ROVI who contributed to your visit. -

MISO Fermentation Class!!!! Organic Growers School – Asheville NC March 2015 Instructor- Liat Batshira : [email protected]
MISO fermentation class!!!! Organic Growers School – Asheville NC March 2015 Instructor- Liat Batshira : [email protected] What is Miso? - fermented soybean paste • Miso is a savory, high protein seasoning made from grain, bean, salt, water and Koji (Aspergillus oryzae culture). • It is a concentrated source of protein, vitamin B12, & other essential nutrients. • It is a live food and has digestion aiding enzymes. • It requires a 2 part fermentation process. Its NOT just a soup! Miso can be used on veggie, meat and egg dished, as spreads and dressings, to ferment veg. pickles, and can even be an ingredient in many desserts! What is Koji? • Grain (typically rice) inoculated with Aspergillus oryzae bacteria. • The bacteria transforms simple sugars of the grain into organic acids which create the flavor and help prevent spoilage. • Can also make Sake (rice wine), Amazake (sweet rice pudding), & Tamari • Japanese translation- “tradition”, “orgin”, “orphan.” Types of miso Aged miso- less koji/more bean, more salt, longer fermentation time, darker color, richer/saltier flavor Barley miso/Mugi-usually red miso. Dark, salty, earthy. White/Sweet miso/Shiro- sweet/mellow flavor. Short fermentation time (~1 month). Good for making spreads, dressings, and desserts. Ranges in color from off white to beige. More koji/less bean, less salt, shorter fermentation time, lighter color Yellow miso/Shinshu- Semi sweet and slightly earthy. Medium fermentation time (~6 months). Ranges in color from beige to yellow/orange. Red miso/Aka- Often made from barley. Long ferment (~1-2 years). Ranges in color from red to dark brown. Black miso/Hatcho- not very common. -

Skagit Valley Food Co-Op Gluten-Free Products Instore Updated 09/11/14
SKAGIT VALLEY FOOD CO-OP GLUTEN-FREE PRODUCTS INSTORE UPDATED 09/11/14 We do our best to keep this list updated, but please check ingredient lists carefully Brand Item Dept. Aisle # Frozen Foods Almond Dream ice cream bites Frozen 1 Amy's Kitchen Black bean Tamale Verde Frozen 1 Amy's Kitchen Rice Macaroni and Cheese Frozen 1 Amy's Kitchen Garden Vegetable Lasagna Frozen 1 Amy's Kitchen Black Bean and Veg. Enchilada, Cheese Enchilada Frozen 1 Amy's Kitchen Sheppard's Pie Frozen 1 Amy's Kitchen Indian Mattar Paneer Frozen 1 Amy's Kitchen Rice Crust Cheese/Spinach Pizza (dairy free) Frozen 1 Amy's Kitchen Light and Lean, Roasted Polenta and swiss chard Frozen 1 Amy's Kitchen Tofu scramble with hash browns & veggies. Frozen 1 Canyon Bakehouse 7 grain bread, hamburger buns Frozen 1 Canyon Bakehouse Rosemary foccacia Frozen 1 Cedarlane Three Layer Enchilada Pie, Garden Veg Enchiladas Frozen 1 Ciao Bella Fruit sorbets Frozen 1 Dr. Praegers Sweet potato pancakes Frozen 1 Evol Bowls Asst. frozen entrees Frozen 1 Food for Life several varieties of GF breads Frozen 1 Food for Life English muffins Frozen 1 Gluten Freeda Asst. frozen burritos, Asst. pizza wraps Frozen 1 Hilarys veggie burger, bean burger Frozen 1 Ians GF fish sticks and chicken nuggets Frozen 1 Jolly Llama Frozen Sorbet Pops Frozen 1 Julie's Organics vanilla ice cream sandwich Frozen 1 Kinnickinnick Pizza Crusts, English muffins Frozen 1 Kinnickinnick frozen waffles Frozen 1 Kinnickinnick cinnamon sugar donuts, vanilla glazed donuts Frozen 1 Kinnickinnick frzn pie crust Frozen 1 Luna and Larry's Coconut Bliss, pints and bars Frozen 1 Nature's Path Asst. -

All About Koji
HORAIYA HONTEN | Booth #47- 47 AMAZAKE (FERMENTED RICE DRINK) Amazake Yogurt (1-2 servings) ALL ABOUT KOJI - RICE MALT Concentrated 100ml Amazake We've heard that this October will mark Since our amazake is non-alcoholic, everyone from Plain Yogurt 80g the 110th anniversary of your company. children to the elderly can enjoy our amazake. Dried Fruits as needed What do you value most with your Cereal as needed Who are your target consumers? products? The health and beauty benefits that amazake has is Quality is the utmost importance at Horaiya. We extraordinary. The koji rice malt breaks down the rice believe that our mission as a food manufacturer is to 1. Cut the dried fruits into small to produce many different enzymes, essential amino pieces. provide high quality products for our customers to acids and vitamins, especially vitamin B. In addition, 2. Mix the amazake, yogurt, and enjoy. For 110 years, we have constantly endeavored koji produces kojic acid, which prevents freckles fruits. to attain our high standards of quality and have been and discoloration of skin, leading to beautiful skin. working to further improve our products. Drinking amazake can be the perfect skin care. The 3. Leave in the refrigerator value of amazake is incredible and we hope to spread A healthy, non-alcoholic overnight. Tell us about the characteristic of your the advantages of drinking amazake to health- 4. Sprinkle cereal on top and enjoy! products. rice beverage full of enzymes, conscious and beauty-conscious consumers abroad. amino acids, and vitamins. Horaiya is a koji manufacturer and we have been Ingredients (2 servings) implementing our traditional koji production What triggered the current amazake Horaiya Honten Co., LTD. -
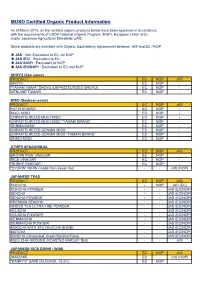
Organic Product List (PDF)
MUSO Certified Organic Product Information As of March 2015, all the certified organic products below have been approved in accordance with the requirements of USDA National Organic Program (NOP), European Union (EC), and/or Japanese Agricultural Standards (JAS). Some products are available with Organic Equivalency Agreements between JAS and EC / NOP. JAS : Non-Equivalent to EC nor NOP JAS (EC) : Equivalent to EC JAS (NOP) : Equivalent to NOP JAS (EC/NOP) : Equivalent to EC and NOP SHOYU (Soy sauce) PRODUCT EC NOP JAS SHOYU EC NOP - "YAMAKI NAMA" SHOYU (UNPASTEURIZED SHOYU) EC NOP - GENUINE TAMARI EC NOP - MISO (Soybean paste) PRODUCT EC NOP JAS HATCHO MISO EC NOP - MUGI MISO EC NOP - UNPASTEURIZED MUGI MISO EC NOP - UNPASTEURIZED MUGI MISO "YAMAKI BRAND“ EC NOP - GENMAI MISO EC NOP - UNPASTEURIZED GENMAI MISO EC NOP - UNPASTEURIZED GENMAI MISO "YAMAKI BRAND“ EC NOP - SHIRO MISO EC NOP - OTHER SEASONINGS PRODUCT EC NOP JAS BROWN RICE VINEGAR EC NOP - RICE VINEGAR EC NOP - "SUSHI" VINEGAR EC NOP - "SYURIN" MIRIN (made from sweet rice) - - JAS (NOP) JAPANESE TEAS PRODUCT EC NOP JAS KUKICHA - NOP JAS (EC) KUKICHA POWDER - - JAS (EC/NOP) SENCHA - - JAS (EC/NOP) SENCHA POWDER - - JAS (EC/NOP) PREMIUM SENCHA - - JAS (EC/NOP) GREEN TEA ULTRA FINE POWDER - - JAS (EC/NOP) HOJICHA - - JAS (EC/NOP) HOJICHA POWDER - - JAS (EC/NOP) GENMAICHA - - JAS (EC/NOP) GENMAICHA POWDER - - JAS (EC/NOP) KUKICHA WITH 20% HOJICHA BLEND - - JAS (EC/NOP) MATCHA - - JAS (EC/NOP) BANCHA (Unroasted, Green Bancha Flake) - - JAS (EC/NOP) MUGI CHA-GROUND (ROASTED BARLEY