Appendix a College of the Rockies Research Ethics Board Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
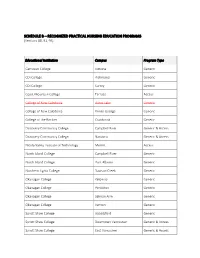
SCHEDULE B – RECOGNIZED PRACTICAL NURSING EDUCATION PROGRAMS (Sections 88, 91, 93) ______
SCHEDULE B – RECOGNIZED PRACTICAL NURSING EDUCATION PROGRAMS (Sections 88, 91, 93) ___________ Educational Institution Campus Program Type Camosun College Victoria Generic CDI College Richmond Generic CDI College Surrey Generic Coast Mountain College Terrace Access College of New Caledonia Burns Lake Generic College of New Caledonia Prince George Generic College of the Rockies Cranbrook Generic Discovery Community College Campbell River Generic & Access Discovery Community College Nanaimo Generic & Access Nicola Valley Institute of Technology Merritt Access North Island College Campbell River Generic North Island College Port Alberni Generic Northern Lights College Dawson Creek Generic Okanagan College Kelowna Generic Okanagan College Penticton Generic Okanagan College Salmon Arm Generic Okanagan College Vernon Generic Sprott Shaw College Abbotsford Generic Sprott Shaw College Downtown Vancouver Generic & Access Sprott Shaw College East Vancouver Generic & Access Educational Institution Campus Program Type Sprott Shaw College Kamloops Generic & Access Sprott Shaw College Kelowna Generic & Access Sprott Shaw College New Westminster Generic & Access Sprott Shaw College Penticton Generic & Access Sprott Shaw College Surrey Generic Sprott Shaw College Victoria Generic Stenberg College Surrey Generic Thompson Rivers University Williams Lake Generic University of the Fraser Valley Chilliwack Generic Vancouver Career College Abbotsford Generic Vancouver Career College Burnaby Generic Vancouver Community College Vancouver (Broadway) Generic & -

Development of a Hospitality Management Program, in the East Kootenay Region Of
1 Development of a Hospitality Management Program, in the East Kootenay Region of British Columbia (Canada), to Address the Employment Needs of the Region Abstract College of the Rockies (Canada), guided by its strategic initiatives, explored the potential of introducing a two-year Hospitality Management Diploma program in Invermere, British Columbia. After a successful feasibility study, the program was developed and introduced during the Spring semester of 2018. The program content is aligned with the provincial core curriculum for Hospitality Management and developed in collaboration with local Hospitality industry inputs. The distinctiveness of the program lies in its design and structure, focused on regional industry employment needs and includes a 500-hour Co-operative education semester to further enhance students’ employability skills and job-readiness for the Hospitality industry. Key words Industry Employability Partnership Collaboration Education Development 2 Introduction Job-ready graduates as well as filling employment gaps are essential focus areas and desired outcomes for tertiary education institutions. College of the Rockies (COTR), British Columbia (BC), Canada explored the local Hospitality industries’ needs to design an academic program to do just that. The Kootenay Regional Skills Training Plan (2013) indicates the demand for workers to increase from 78 560 in 2012 to 83 240 in 2020, thus predicting a 5.6% increase, as indicated in Figure 1.1 Kootenay Labour Demand and Supply Projections 2012 to 2020. The Kootenay Regional Skills Training Plan (2013) further reveals the shortage of qualified employees in the Tourism and Hospitality industries in the Kootenay region. The region, with an already shortfall of qualified employees, has a projected Tourism and Hospitality employee growth, potentially similar to the 1.6% estimated for the province as well as business development that support the claim of a desired increase of qualified employees. -

Camosun College Transportation and Parking Management Plan
Camosun College Transportation and Parking Management Plan By Todd Litman Victoria Transport Policy Institute 2009 Revised June, 2009 Camosun College Transportation and Parking Management Plan Victoria Transport Policy Institute Camosun College Transportation and Parking Management Plan 18 June 2009 By Todd Litman Victoria Transport Policy Institute Summary The Camosun College Transportation and Parking Management (TPM) Project includes a planning process to identify optimal solutions to campus transportation and parking problems. Through the TPM project, specific ways to improve transportation and parking management in order to create a more sustainable campus will be identified. The plan will be flexible and responsive to future demands and conditions. This TPM plan describes existing transportation and parking conditions, identifies current and future challenges, and recommends specific transportation and parking policies and management programs. The TPM Project will continue beyond this plan through the implementation phases. 2 Camosun College Transportation and Parking Management Plan Victoria Transport Policy Institute Contents Introduction .................................................................................................................................................... 5 Planning Goals and Objectives ....................................................................................................................... 5 Camosun College Campuses .......................................................................................................................... -

Agent Profile Company Name
International Education 100 West 49th Avenue Vancouver, B.C. CANADA V5Y 2Z6 Agent Profile Company Name Address City State/Prov/Pref Country Postal Code Telephone Fax Company Web Site Signing Officer’s Name Signing Officer’s Title Contact Person’s Name Contact Person’s Title Contact Person’s E-mail Agent Questions 1. What is the purpose of your company? 2. How long have you been an agent for overseas educational institutions? Langara College 3. What other recruiting agencies, companies or partners do you work with? 4. Which schools do you currently have contracts with? Alexander College Centennial College B.C.I.T. Conestoga College Camosun College Durham College Capilano University Fanshawe College College of the Rockies George Brown College Columbia College Georgian College Coquitlam College Humber College Douglas College Mohawk College Fraser International College (FIC) Seneca College Kwantlen Polytechnic University Sheridan College North Island College Mount Saint Vincent University Okanagan College Mount Alison University Simon Fraser University (SFU) Queens University Thompson Rivers University (TRU) York University Trinity Western University (TWU) University of Alberta University of the Fraser Valley (UFV) University of Calgary University of Northern British Columbia University of Manitoba (UNBC) University of New Brunswick University of Victoria (UVic) University of Saskatchewan Vancouver Community College (VCC) University of Western Ontario Vancouver Island University (VIU) University of Windsor British Columbia School Districts: -

Biographical Notes
BC Quality Assurance Process Audit Experience: Context and Initial Lessons Joseph & Rosalie Segal Centre 515 W Hastings Street SFU Harbour Centre Vancouver, BC V6B 5K3 Biographical Notes Ronald Bond, Keynote Speaker Ronald Bond is the former Provost and Vice-President Academic (Emeritus) at the University of Calgary, where he was also a Professor of English and the Dean of Humanities. He was Chair of the Campus Alberta Quality Council for two terms, has served as the external “expert” of the Quality Council of the Council of Ontario Universities, and belongs to the Organizational Review Committee of Ontario’s Postsecondary Education Quality Assessment Board. He is currently Chair of the Saskatchewan Higher Education Quality Assurance Board. He has extensive experience as a consultant and as a facilitator of workshops on quality assurance in higher education. In addition to participating on c. 20 organizational and program review panels in various jurisdictions in Canada, he had conducted (with Sam Scully) assessments of the QA functions of the Maritimes Provinces Higher Education Council for the Council of Ministers of Education and Training in the three Atlantic provinces (2014) and a government-sponsored review, of the Campus Alberta Quality Council (2017). He was also pleased to be a utility infielder on the “Stubbs” committee set up by DQAB to review and offer advice on elements of the QA system in B.C. (2011.) Panelists Karen Belfer oversees the self-regulatory mechanisms for the public colleges in Ontario and is responsible for the operation of the Credentials Validation Service and the College Quality Assurance Audit Process. -

Engineering Common Curriculum Project Update
For more information, please contact Brian Case, BCcampus Project Manager at [email protected] Engineering Common Curriculum Project Update Update: Fall 2020 Completed Tasks Nov 2019: Five course shells created and shared on BCCAT Moodle site. Project Overview Eleven funding agreements were created to assist help the following institutions move to the common curriculum: In Spring 2019, BCcampus was asked by the Ministry of Advanced Education, Skills and Training to assist with a project concerning B.C. institutions delivering • Capilano University engineering programs who want to move to a common first-year curriculum. • Coast Mountain College The project was initiated by the Engineering Articulation Committee and led by • College of New Caledonia Brian Dick of Vancouver Island University. The work conducted to determine the feasibility of a common first-year curriculum was initially funded through a grant • College of the Rockies from the B.C. Council on Admissions and Transfer. • Langara College • North Island College Project Objectives • Northern Lights College • Selkirk College • Improve access to and opportunity for success in engineering education for • Thompson Rivers University B.C.’s diverse post-secondary learners • Vancouver Community College • Create opportunities for regional community engagement and partnerships • Vancouver Island University within the engineering sector, encouraging graduates to return to employment These institutions are at various stages of in smaller, non-urban communities implementation, with the expectation that • Enhance the student learning environment and improve retention and all will be aligned by the 2021/22 academic achievement in engineering across the province through maximizing use of year (impact of COVID-19 not yet deter- student supports, class size, and regional diversity mined). -

The Definition of Athletics Representative
Camosun College Intercollegiate Athletics COACH HANDBOOK Updated August 2019 CAMOSUN COLLEGE CHARGERS COACH HANDBOOK TABLE OF CONTENTS Page Welcome 3 Chargers Athletics Vision, Mission and Values 4 PISE Welcome and Policies 5 Camosun College Organizational Chart 6 Centre for Sport and Exercise Education Organizational Chart 7 Section 1. PACWEST & CCAA Membership 8 Section 2. Camosun Chargers Athletics Department Contacts 9 Section 3. Facilities 10 Section 4. Objectives of the Chargers Program 10 Section 5. Our Commitment 11 Section 6. Coaching Responsibilities & Duties 12 - 15 Section 7. Student-Athlete Eligibility 16 Section 8. Operation of the Chargers Program 16 - 18 Section 9. Travel Policies 18 - 19 Section 10. Financial Assistance 20 Section 11. Intercollegiate Athletic Awards 20 - 22 Appendix A. Coaching Code of Conduct 23 - 24 Appendix B. Fair Play Codes 25 Appendix C. CAC Coaching Code of Conduct 26 Appendix D. CCAA Coach Consent Form 27 Appendix E. Important Website Addresses 28 Appendix F. CCES Quick Reference Card 29 Appendix G. Camosun Student-Athlete Experience Evaluation 30 Chargers Coaches Handbook revised 07/16 2 Welcome On behalf of the Centre for Sport and Exercise Education (CSEE) and the Recreation and Athletics department, I am pleased to welcome you to Camosun College and the Chargers Intercollegiate Athletics program. This Chargers Coach Handbook has been developed by the Athletics department to assist you with the successful operation of your program. It contains useful information about PACWEST, facilities, coaching responsibilities, student-athlete policies and procedures and student support services available at Camosun. Please take time to review and become familiar with the information contained in this handbook. -

ASE Program Specific Transfer Guide Project
Adult Special Education (ASE) Program-Specific Transfer Guide Project Contractor: Donna Lowndes, Douglas College Articulation Committee Co-Chairs: Kimberly McIntyre, Northwest Community College Alison Roy, Selkirk College May 2018 1 Introduction: In British Columbia’s public post-secondary institutions, Adult Special Education (ASE) programs respond to the needs of a diverse group of learners. Individuals with disabilities, or with a combination of barriers to education, employment or independence, as described in Appendix A, are eligible to enroll in these programs/courses in accordance with each institution’s guidelines (Douglas College, 2009). ASE programs also respond to industry and community needs, and relate directly to local labour market trends. 15 BC post-secondary institutions offer ASE courses and programs. The topics in ASE programs and courses include, but are not limited to, skills that increase independence, literacy and numeracy, computer literacy, employment transition, employment readiness, and vocational skills training. Learning is enhanced by the use of student-centered best practices. ASE programs and courses emphasize skill development for the workplace, and promote independence, community inclusion, and lifelong learning. The purpose of the ASE Program-Specific Transfer Guide is to provide information to learners, parents, caregivers, instructors, employers, community agencies and counselors throughout British Columbia regarding the purpose of ASE articulation, learning outcomes for general program type offerings and a table of programs offered at each institution. The Guide will help its users with program awareness and identify transferability among ASE Employment Readiness Programs, many of which are not eligible for inclusion in the BC Transfer Guide because they are categorized as developmental or preparatory courses. -

The Definition of Athletics Representative
Camosun College Intercollegiate Athletics COACHES HANDBOOK 2013 – 2014 Updated August 2013 CAMOSUN COLLEGE CHARGERS COACHES’ HANDBOOK TABLE OF CONTENTS Page Welcome and Introduction 3 Chargers Vision, Mission and Values 4 PISE Welcome and Policies 5 College Organization 6 Recreation & Athletics Department Organization 7 Section 1. PACWEST & CCAA Membership 8 Section 2. Camosun College Contacts 9 Section 3. Facilities 10 Section 4. Objectives of the Chargers Program 11 Section 5. Our Commitment 11 - 12 Section 6. Coaching Responsibilities & Duties 12 - 16 Section 7. Student-Athlete Eligibility 16 - 27 Section 8. Operation of the Chargers Program 28 - 29 League Schedules 28 Exhibition Schedules 28 Department Funding 28 Athletics Budget 28 Fundraising 28 Uniforms & Equipment 29 Important Chargers Events 29 Student-Athlete Grievance Procedure 29 Section 9. Travel Policies 30 - 31 Section 10. Financial Assistance 32 Section 11. Intercollegiate Athletic Awards 32 - 34 Appendix A. CABC Coaching Code of Conduct 35 - 36 Appendix B. CABC Fairplay Codes 37 - 39 Appendix C. CAC Coaching Code of Ethics 40 - 46 Appendix D. Coach Consent Form 47 Appendix E. Important Website Addresses 48 Appendix F. CCES Quick Reference Card (2009) 49 Appendix G. LifeMark Sport Medicine Services 50 - 51 Chargers Coaches Handbook 2012-2013 2 Welcome from the Recreation and Athletics Department On behalf of the Recreation and Athletics department, I am pleased to welcome you to Camosun College and the Chargers Intercollegiate Athletics program. This Chargers Coaches Handbook has been developed by the Recreation and Athletics department to assist you with the successful operation of your program. It contains useful information about PACWEST, facilities, coaching responsibilities, student-athlete policies and procedures and student support services available at Camosun. -

Bc Simulation Current State Report
Provincial Simulation Coordination Committee Current State Report BC SIMULATION CURRENT STATE REPORT Prepared by: BC Provincial Simulation Coordination Committee Delivered to: BC Ministry of Advanced Education, Innovation, and Technology BC Ministry of Health November 29, 2013 November 19, 2013 Page 0 of 35 Provincial Simulation Coordination Committee Current State Report 1 Executive Summary The Provincial Simulation Coordination Committee (PSCC) was established in June 2012 and functions as a central coordinating and advisory organization. The PSCC’s goal is to support health authorities and health professional education institutions province wide to advance the efficient development of simulation education through an integrated approach that improves access to simulation facilities, technologies, and resources. In early 2013, the PSCC received funding to develop a Simulation Roadmap for BC. The first deliverable in this roadmap is a Current State Report that aims to enable the BC Government to make effective, contextual decisions on where to allocate taxpayer funds based on an improved understanding of the current simulation environment. A PSCC Sub-Committee was established to lead the development of the Current State Report and included representatives from UBC, BCIT, and Northern Health Authority. The Sub-Committee developed a list of online survey questions for stakeholders identified by the PSCC across the Province according to regional, professional, and institutional affiliation. Approximately 80 individuals were invited to complete the survey on May 27, 2013 and 56 completed responses were received by the survey close on July 5, 2013 for a 70% completion rate. Key findings from the survey were grouped into three categories: 1. Equipment and Technology 2. -

Camosun College Board of Governors Regular Meeting Agenda October 13, 2020
BOARD OF GOVERNORS REGULAR MEETING AGENDA MEETING: Tuesday, October 13, 2020 TIME: 5:00 pm ONLINE: Teams BOARD MEMBERS: ADMINISTRATION: Monty Bryant, Chair John Boraas, VP Education Bijan Ahmadi Heather Cummings, VP Student Experience Sherri Bell, President Deborah Huelscher, VP Administration & CFO Tanya Clarmont Rodney Porter, Exec. Dir., Communications & Marketing Joanne Cumberland Barbara Severyn, Exec. Dir., Human Resources Richard Margetts Geoff Wilmshurst, VP Partnerships Brenda McBain Brent Palmer GUEST: Scott Harris, Registrar Margie Parikh Emily Rogers, Vice Chair REGRETS: nil Mike Stubbing Al van Akker EXECUTIVE ASSISTANT: Heather Martin Fillette Umulisa Lindsay JD van Gerven Phil Venoit Camosun College campuses are located on the Traditional Territories of the Lekwungen and W̱ SÁNEĆ peoples. We acknowledge their welcome and graciousness to the students who seek knowledge here. I CALL TO ORDER PAGE II APPROVAL OF THE AGENDA III BOARD MEMBER REPORTS 1. Chair’s Report [5 min] (Monty Bryant) no attachment 2. President’s Report [5 min] (Sherri Bell) no attachment 3. Foundation [5 min] (Tanya Clarmont/Geoff Wilmshurst) no attachment 4. Education Council [5 min] (Bijan Ahmadi/Joanne Cumberland) i) Minutes of the June 24, 2020 Special & Regular meetings attachment 4 5. Pacific Institution for Sport Excellence [5 min] (Phil Venoit) no attachment 6. Financial Update [10 min] (Mike Stubbing, Deborah Huelscher) no attachment Quorum: Majority Voting Members Page 1 of 2 Camosun College Board of Governors Regular Meeting Agenda October 13, 2020 PAGE IV BOARD COMMITTEE REPORTS 1. Executive Committee [5 min] (Monty Bryant) no attachment i) Public Sector Employers’ Council Secretariat (PSEC): Executive Compensation Freeze [10 min] (Monty Bryant) * attachment 9 V APPROVAL OF THE MINUTES 1. -

Find out More
ADVENTURE TOURISM BUSINESS OPERATIONS CERTIFICATE AND DIPLOMA PROGRAM Information Guide CONTACT US 1305 - 9 Street S., PO Box 376 Golden, BC V0A 1H0 250-344-5901 [email protected] WELCOME Welcome Thanks for your interest in the Adventure Tourism Business Operations (ATBO) certificate and diploma program. This program has been developed to help you convert your passion for outdoor adventure into an exciting and rewarding career. The ATBO program personifies the Rocky Mountain way of life, taking advantage of the many adventures available on our doorstep. World-class ski hills, white-water rivers, hiking, and mountain biking trails often serve as your classroom. Our collaboration with industry professionals means you have the opportunity to learn from some of the best in the business. Innovative curriculum in both the classroom and the natural environment can prepare you to excel as an adventure tourism professional. Much like the many routes available in the backcountry, the Adventure Tourism Business Operations graduate has numerous potential paths they may follow, each with its own unique benefits and rewards. Our students have gone on to work and thrive in jobs all over the globe, in settings as diverse as mountain peaks and white-water rivers, management of a boutique hotel, and a kangaroo sanctuary off Australia’s south coast. No matter what path you chose to take, you can expect to bring a balance of highly-applicable and transferable skills with you. TABLE OF CONTENTS Table of Contents About the Program ..............................................................................................................................2