20210806095625645 IU Vax Appendix Final For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hair: the Performance of Rebellion in American Musical Theatre of the 1960S’
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Winchester Research Repository University of Winchester ‘Hair: The Performance of Rebellion in American Musical Theatre of the 1960s’ Sarah Elisabeth Browne ORCID: 0000-0003-2002-9794 Doctor of Philosophy December 2017 This Thesis has been completed as a requirement for a postgraduate research degree of the University of Winchester MPhil/PhD THESES OPEN ACCESS / EMBARGO AGREEMENT FORM This Agreement should be completed, signed and bound with the hard copy of the thesis and also included in the e-copy. (see Thesis Presentation Guidelines for details). Access Permissions and Transfer of Non-Exclusive Rights By giving permission you understand that your thesis will be accessible to a wide variety of people and institutions – including automated agents – via the World Wide Web and that an electronic copy of your thesis may also be included in the British Library Electronic Theses On-line System (EThOS). Once the Work is deposited, a citation to the Work will always remain visible. Removal of the Work can be made after discussion with the University of Winchester’s Research Repository, who shall make best efforts to ensure removal of the Work from any third party with whom the University of Winchester’s Research Repository has an agreement. Agreement: I understand that the thesis listed on this form will be deposited in the University of Winchester’s Research Repository, and by giving permission to the University of Winchester to make my thesis publically available I agree that the: • University of Winchester’s Research Repository administrators or any third party with whom the University of Winchester’s Research Repository has an agreement to do so may, without changing content, translate the Work to any medium or format for the purpose of future preservation and accessibility. -

News Briefs the Elite Runners Were Those Who Are Responsible for Vive
VOL. 117 - NO. 16 BOSTON, MASSACHUSETTS, APRIL 19, 2013 $.30 A COPY 1st Annual Daffodil Day on the MARATHON MONDAY MADNESS North End Parks Celebrates Spring by Sal Giarratani Someone once said, “Ide- by Matt Conti ologies separate us but dreams and anguish unite us.” I thought of this quote after hearing and then view- ing the horrific devastation left in the aftermath of the mass violence that occurred after two bombs went off near the finish line of the Boston Marathon at 2:50 pm. Three people are reported dead and over 100 injured in the may- hem that overtook the joy of this annual event. At this writing, most are assuming it is an act of ter- rorism while officials have yet to call it such at this time 24 hours later. The Ribbon-Cutting at the 1st Annual Daffodil Day. entire City of Boston is on (Photo by Angela Cornacchio) high alert. The National On Sunday, April 14th, the first annual Daffodil Day was Guard has been mobilized celebrated on the Greenway. The event was hosted by The and stationed at area hospi- Friends of the North End Parks (FOTNEP) in conjunction tals. Mass violence like what with the Rose F. Kennedy Greenway Conservancy and North we all just experienced can End Beautification Committee. The celebration included trigger overwhelming feel- ings of anxiety, anger and music by the Boston String Academy and poetry, as well as (Photo by Andrew Martorano) daffodils. Other activities were face painting, a petting zoo fear. Why did anyone or group and a dog show held by RUFF. -

AXS TV Schedule for Mon. June 29, 2020 to Sun. July 5, 2020 Monday
AXS TV Schedule for Mon. June 29, 2020 to Sun. July 5, 2020 Monday June 29, 2020 7:20 PM ET / 4:20 PM PT 8:00 AM ET / 5:00 AM PT AXS TV Insider Rock Legends Featuring highlights and interviews with the biggest names in music. Earth, Wind & Fire - Earth, Wind & Fire is an American band that has spanned the musical genres of R&B, soul, funk, jazz, disco, pop, rock, Latin and African. They are one of the most successful 7:30 PM ET / 4:30 PM PT bands of all time. Leading music critics cast fresh light on their career. Rock Legends Journey - Journey is an American rock band that formed in San Francisco in 1973, composed of 8:30 AM ET / 5:30 AM PT former members of Santana and Frumious Bandersnatch. The band has gone through several Rock & Roll Road Trip With Sammy Hagar phases; its strongest commercial success occurred between 1978 and 1987. Sunset Strip - Sammy heads to Sunset Blvd to reminisce at the Whisky A-Go-Go before visit- ing with former Mötley Crüe drummer Tommy Lee at his house. After cooking together and Premiere exchanging stories, the guys rock out in Tommy’s studio. 8:00 PM ET / 5:00 PM PT Nothing But Trailers 9:00 AM ET / 6:00 AM PT Sometimes the best part of the movie is the preview! Watch some of the best trailers, old and The Big Interview new, during this special presentation. Dwight Yoakam - Country music trailblazer takes time from his latest tour to discuss his career and how he made it big in the business far from Nashville. -
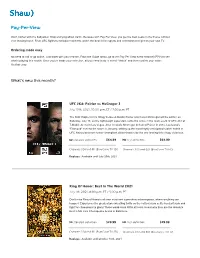
Pay-Per-View
Pay-Per-View Don’t bother with the babysitter. Stop worrying about traffic. Because with Pay Per View, you get the best seats in the house without ever leaving home. From UFC fights to exclusive concerts, watch the best in live sports and entertainment right on your own TV. Ordering made easy No need to call or go online. Just order with your remote. From the Guide menu, go to the Pay Per View event channel (PPV) to see what’s playing this month. Once you’ve made your selection, all you need to do is select “Watch” and then confirm your order. It’s that easy. What’s new this month? UFC 264: Poirier vs McGregor 3 July 10th, 2021, 10:00 p.m. ET / 7:00 p.m. PT The final chapter in the trilogy between Dustin Poirier and Conor McGregor will be written on Saturday, July 10, as the lightweight superstars settle the score in the main event of UFC 264 at T-Mobile Arena in Las Vegas. After Ireland's McGregor defeated Poirier in 2014, Louisiana's "Diamond" evened the score in January, setting up the most highly anticipated rubber match in UFC history between former champions determined to be the one leaving this trilogy victorious. SD standard definition $64.99 HD high definition $64.99 Channels 324 and 611 (BlueCurve TV SD) Channels 300 and 601 (BlueCurve TV HD) Replays: Available until July 25th, 2021 Ring Of Honor: Best In The World 2021 July 11h, 2021, 8:00 p.m. ET / 5:00 p.m. -

Ajaja Gbara Eni : Exploring Citizen Rebellion in Post
AJAJA GBARA ENI1: EXPLORING CITIZEN REBELLION IN POST-COLONIAL NIGERIA Elom Tettey-Tamaklo Senior Thesis, 2019 Department of Political Science, Haverford College Dr. Susanna Wing, Advisor 1 Fighting Oppression/Liberation Struggles 1 TABLE OF CONTENTS Acknowledgements………………………………………………………………………….3 Introduction and Methodology ……………………………………………………………..4 The Post Colonial African Project ………………………………………………….5 CHAPTER 1: Literature Review …………………………………………………………..10 A: Theories of Resistance ………………………………………………………….12 B: Forms of Resistance …………………………………………………………….14 CHAPTER 2: Roots of Rebellion …………………………………………………………..28 Family Legacy ………………………………………………………………………28 Yoruba Influences …………………………………………………………………. 32 CHAPTER 3: The State of The State………………………………………………………..43 Features of the Post-Colonial Nigerian Project……………………………………...47 CHAPTER 4 : Bridge Over Troubled Waters…………………………………………….....54 A: Civil Society ……………………………………………………………………..54 B: The Rise of Lone Wolf Citizen Activism………………………………………...62 CHAPTER 5: Fela’s Rebellion ……………………………………………………………..64 Fela: The Political Philosopher ……………………………………………………..67 Fela: The Political Griot …………………………………………………………….74 Fela: The Political Practitioner ……………………………………………………...94 Conclusion …………………………………………………………………………………102 2 ACKNOWLEDGEMENTS A popular African proverb says, “if you want to go fast, go alone, but if you want to go far, go together.” The journey of writing this thesis has been a long and hard road and it would not have been possible without the help of the following people. First of all, I want to thank my parents who have supported me through this process. Your late night calls, encouraging texts and words of affirmation made me believe that I could do it. Also, I thank my tribe; my family and close friends in and outside Haverford College who have helped me write this thesis. You have encouraged me, checked up on me, read my drafts with me, stayed through the night with me, prayed for me and believed in me. -

Liquid Blackness Reflects on the Expansive Possibilities of the L.A
Georgia State University ScholarWorks @ Georgia State University Communication Faculty Publications Department of Communication 2015 Encountering the Rebellion: Liquid Blackness Reflects on the Expansive Possibilities of the L.A. Rebellion Films Alessandra Raengo Georgia State University, [email protected] Follow this and additional works at: https://scholarworks.gsu.edu/communication_facpub Part of the Communication Commons Recommended Citation Alessandra Raengo. “Encountering the Rebellion: liquid blackness reflects on the expansive possibilities of the L.A. Rebellion films,” in L.A. Rebellion: Creating a New Black Cinema, ed. Allyson Nadia Field, Jan- Christopher Horak, Jacqueline Stewart, pp. 291-318. University of California Press, 2015. This Book Chapter is brought to you for free and open access by the Department of Communication at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Communication Faculty Publications by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. L.A. Rebellion Creating a New Black Cinema E D IT E D BY Allyson Nadia Field, Jan-Christopher Horak, and Jacqueline Najuma Stewart 15 UNIVERSITY OF CALIFORNIA PRESS University of California Press, one of the most distinguished university presses in the United States, enriches lives around the world by advancing scholarship in the humanities, social sciences, and natural sciences. Its activities are supported by the UC Press Foundation and by philanthropic contributions from individuals and institutions. For more information, visit www .ucpress.edu. University of California Press Oakland, California © 2015 by The Regents of the University of California Library of Congress Cataloging-in-Publication Data L.A. Rebellion : creating a new black cinema/edited by Allyson Nadia Field, Jan-Christopher Horak, and Jacqueline Najuma Stewart, pages cm Includes bibliographical references and index. -

Resistance, Rebellion and Revolt!
OCIALiT SCHOLARS CONFERENCE Resistance, Rebellion and Revolt! April 18, 19 & 20, 1986 The 4th Annual Boro of Manhattan Community College, CUNY, 199 Chambers Street, New York City FRIDAY SATURDAY SUNDAY 7-10 pm 9:30 am-lO pm 9:30 am-6 pm Resistance, Rebellion and Revolt! A broad range of academics and social activists have gathered for a 4th Annual Socialist Scholars Conference on Friday through Sunday, April 18-20 at the Boro of Manhattan Community College. This year's theme of Resistance, Rebellion and Revolt focuses upon a century of mass workers movements: 100th Anniversary of the Haymarket Riots, 75th Anniversary of the Triangle Shirtwaist Factory Fire (which helped galvanize the women's working-class movement here in New York City), the 50th Anniversary of the great sit-down strikes which helped found the CIO, the 50th Anniversary of the Spanish Civil War, and the 30th Anniversary of the Hungarian Revolt. This year we are commemorating the millions who have fought and died in the real socialist struggle, the continual revolt from below. CHILDCARE AVAILABLE Saturday 9:00 am - 5:00 pm Sunday 9:00 am - 5:00 pm Children must toilet trained and at least three years old. ROOM N312 The Graduate School and University Center of the City University of New York Ph.D. Program in Sociology I Box 375 Graduate Center: 33 West 42 Street, New York, N.Y. 10036-8099 212 790-4320 To Our Fellow Participants: Socialism, in the multifaceted forms it has adopted, has inspired a vast number of social movements in a wide range of countries. -

Taiping Pipe Dreams: Women's Roles in The
Taiping Pipe Dreams: Women’s Roles in the Taiping Rebellion A Senior Honors Thesis Presented in Partial Fulfillment of the Requirements for graduation with distinction in History in the undergraduate colleges of The Ohio State University by Adrienne Johnson The Ohio State University June 2006 Project Advisor: Professor Cynthia Brokaw, Department of History Contents: Introduction – 1 Rules for Women in Imperial China - 5 Women in the Qing - 12 Origins of the Taiping Rebellion – 17 The Taiping Social Program – 23 Women’s Lives Under the Taiping – 27 Conclusion - 36 1 A twenty-year-old woman is hunched over, digging a ditch in the hot sun. She stands unsteadily on her feet, a mere four inches in length, stunted and gnarled from years of being tightly bound. The cloths that cramped their growth since she was a child have now been removed. This is the first manual labor she has done in her life; any work before this day had been within the home, cooking, cleaning, weaving, caring for the children. In fact, it is the first time she has been outside in the last ten years, save only for holidays and very special occasions. The army that has invaded her village tells her that she is now equal to men. She may go outside when she wishes, she may work in jobs previously delegated only to men, she may own property. Years of oppression have been lifted from her shoulders. She is free. Or is she? Yes, she stands outside when her life before had been cloistered within a household, but what on what does she stand? Feet so small that she has difficulty walking, let alone digging a ditch. -

Resilience Beyond Rebellion: How Wartime Organizational Structures Affect Rebel-To-Party Transformation
Resilience Beyond Rebellion: How Wartime Organizational Structures Affect Rebel-to-Party Transformation By Sheryl Zaks A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Political Science in the Graduate Division of the University of California, Berkeley Committee in charge: Professor David Collier, Co-chair Professor Ron Hassner, Co-chair Professor Leonardo Arriola Professor Heather A. Haveman Summer 2017 Abstract Scholars have established that the best prospects for long-term stability and democra- tization in war-torn states occur when former rebels compete in post-conflict elections. However, only half of the insurgencies with political aspirations successfully reinvent themselves as lasting opposition parties. Why are some rebel groups able to seamlessly transition into political parties while others revert to violence or die trying? My disser- tation draws on insights from organizational sociology to model the process and risks of rebel-to-party transition. I identify three wartime domains that I call proto-party struc- tures: shadow governance, political messaging, and social service wings. Proto-party structures represent a surprising dimension of rebel organizational diversity. Crucially, however, not all insurgencies have them. I demonstrate that these structures|by mirror- ing the key components of political party organizations|provide insurgencies with two decisive advantages when attempting to transition into a party: (1) relevant experience that translates into the political arena, and (2) an easier path to transition by repurpos- ing existing structures rather than building a party from scratch. I use a mixed-method approach|combining statistical analyses on a novel dataset with process tracing in three cases|to test my organizational theory of transition. -

Complete Undergraduate Catalog
2021-2022 Undergraduate Catalog Table of Contents Disclaimer on Content Change.................................................................................. 15 Alfred At a Glance......................................................................................................16 Profile...................................................................................................................16 Founded........................................................................................................16 Type of institution.........................................................................................16 Location........................................................................................................ 16 Students........................................................................................................16 Campus........................................................................................................ 16 Admissions................................................................................................... 16 Financial Aid.................................................................................................16 Graduates..................................................................................................... 16 Academics........................................................................................................... 17 Programs...................................................................................................... 17 Class -

May 2019 Title Grid Event TV Title Grid - Version 4 4/23/2019 Approx Length Final Events Distributor Genre (Hours) Preshow Premiere Exhibition SRP Rating
May 2019 Title Grid Event TV Title Grid - Version 4 4/23/2019 Approx Length Final Events Distributor Genre (hours) Preshow Premiere Exhibition SRP Rating AEW: Double or Nothing LIVE (Live) XTV: Xtreme Television Wrestling 5 Y 05/25/19 05/25/19 $49.95 TV-14 AEW: Double or Nothing LIVE (Replay) XTV: Xtreme Television Wrestling 4 N 05/27/19 05/30/19 $49.95 TV-14 AXS TV Fights: Legacy Fighting Alliance 60 AXS TV Mixed Martial Arts 2.5 N 05/17/19 05/31/19 $9.95 TV-14 AXS TV Fights: Legacy Fighting Alliance 61 AXS TV Mixed Martial Arts 3 N 05/31/19 05/31/19 $9.95 TV-14 Back Alley Brawlers Exposed Stonecutter Media Uncensored TV 1 N 05/05/19 05/31/19 $9.95 TV-14 Code Red: Half Baked Videos XTV: Xtreme Television Uncensored TV 1 N 05/10/19 05/31/19 $9.95 TV-14 Devo: Hardcore Devo Live MVD Entertainment Group Music / Concert 1.5 N 05/03/19 05/30/19 $5.95 TV-14 Extreme Legends: Terry Funk Stonecutter Media Wrestling 1 N 05/12/19 05/31/19 $7.95 TV-14 Female Wrestling's Most Violent Brawls 66 The Wrestling Zone, Inc. Wrestling 1 N 05/22/19 05/31/19 $7.95 TV-14 Rhythm 'n' Bayous: A Road Map to Louisiana Music MVD Entertainment Group Documentary / Music 2.5 N 05/04/19 05/31/19 $5.95 TV-14 ROH LI: Castle vs. Scurll XTV: Xtreme Television Wrestling 1 N 05/23/19 05/31/19 $9.95 TV-14 The Roast of Ric Flair: Live & Uncensored (Live) XTV: Xtreme Television Comedy 2.5 N 05/24/19 05/24/19 $19.95 TV-MA The Roast of Ric Flair: Live & Uncensored (Replay) XTV: Xtreme Television Comedy 2.5 N 05/26/19 05/30/19 $19.95 TV-MA Timothy Leary's Dead MVD Entertainment Group Documentary 1.5 N 05/03/19 05/30/19 $4.95 TV-14 UFC 237: TBD vs. -

AXS TV Canada Schedule for Mon. July 27, 2020 to Sun. August 2, 2020
AXS TV Canada Schedule for Mon. July 27, 2020 to Sun. August 2, 2020 Monday July 27, 2020 4:00 PM ET / 1:00 PM PT 6:00 AM ET / 3:00 AM PT The Top Ten Revealed Tom Green Live Live Albums - Live albums can define a band. Today we are ranking live albums that got the Andrew Dice Clay - Seriously. Funny. Talk. from comedian/actor Tom Green and fellow comedian/ crowds rockin’! Find out which ones make our list as rock experts Rikki Rockett, Dee Snider, and actor Andrew Dice Clay! Steven Adler count us down! 7:00 AM ET / 4:00 AM PT 4:30 PM ET / 1:30 PM PT The Very VERY Best of the 70s The Day The Rock Star Died Horror Films - From gory to heart-stopping, these films had pulses racing in the 70s. Find out Amy Winehouse - Amy Winehouse was an English singer and songwriter whose stunning voice which 70s horror films made our list as Catherine Bach, George Wallace, Morgan Fairchild, Anson easily followed in the footsteps of Janis Joplin and Billie Holiday. Her debut album, Frank, was a Williams, and more give us their opinions! critical success in the UK and was nominated for the Mercury Prize. Her follow-up album, Back to Black, led to five 50th Annual Grammy Awards, and a record of most Grammys won by a female 7:30 AM ET / 4:30 AM PT artist in one night. Her album Back to Black posthumously became one the UK’s best-selling TrunkFest with Eddie Trunk albums of the 21st century.