Population-Based Serosurvey for Severe Acute Respiratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

15 Sub Ptt MSB-TBM-CGL DOWN WEEK DAYS
CHENNAI BEACH - TAMBARAM - CHENGALPATTU DOWN WEEK DAYS Train Nos 40501 40001 40503 40505 40507 40701 40509 Kms Stations CJ 0 Chennai Beach d 03:55 04:15 04:35 04:55 05:15 05:30 05:50 2 Chennai Fort d 03:59 04:19 04:39 04:59 05:19 05:34 05:54 4 Chennai Park d 04:02 04:22 04:42 05:02 05:22 05:37 05:57 5 Chennai Egmore d 04:05 04:25 04:45 05:05 05:25 05:40 06:00 7 Chetpet d 04:08 04:28 04:48 05:08 05:28 05:43 06:03 9 Nungambakkam d 04:11 04:31 04:51 05:11 05:31 05:46 06:06 10 Kodambakkam d 04:13 04:33 04:53 05:13 05:33 05:48 06:08 12 Mambalam d 04:15 04:35 04:55 05:15 05:35 05:50 06:10 13 Saidapet d 04:18 04:38 04:58 05:18 05:38 05:53 06:13 16 Guindy d 04:21 04:41 05:01 05:21 05:41 05:56 06:16 18 St.Thomas Mount d 04:24 04:44 05:04 05:24 05:44 05:59 06:19 19 Palavanthangal d 04:27 04:47 05:07 05:27 05:47 06:02 06:22 21 Minambakkam d 04:30 04:50 05:10 05:30 05:50 06:05 06:25 22 Tirusulam d 04:32 04:52 05:12 05:32 05:52 06:07 06:27 24 Pallavaram d 04:35 04:55 05:15 05:35 05:55 06:10 06:30 26 Chrompet d 04:38 04:58 05:18 05:38 05:58 06:13 06:33 29 Tambaram Sanatorium d 04:41 05:01 05:21 05:41 06:01 06:16 06:36 30 Tambarm a 05:10 d 04:50 05:30 05:50 06:10 06:25 06:45 34 Perungulathur d 04:56 05:36 05:56 06:16 06:32 06:56 36 Vandalur d 04:59 05:39 05:59 06:19 06:35 06:59 39 Urappakkam d 05:03 05:43 06:03 06:23 06:39 07:03 42 Guduvancheri d 05:07 05:47 06:07 06:27 06:43 07:07 44 Potheri d 05:11 05:51 06:11 06:31 06:47 07:11 46 Kattangulathur d 05:14 05:54 06:14 06:34 06:50 07:14 47 Maraimalai Nagar d 05:16 05:56 06:16 06:36 06:52 07:16 51 Singaperumal -

Heavy Vehicles Factory, Avadi, Chennai Heavy Vehicles Factory, Avadi, Chennai Scheme of Presentation
HEAVY VEHICLES FACTORY, AVADI, CHENNAI HEAVY VEHICLES FACTORY, AVADI, CHENNAI SCHEME OF PRESENTATION • About HVF and its products • Opportunities in HVF • Challenges in Indigenization. • Process of procurement. HEAVY VEHICLES FACTORY, AVADI, CHENNAI PRINCIPAL PRODUCTS 1. T-90S TANKS 2. ARJUN TANKS 3. OVERHAULING OF T-72 TANKS HEAVY VEHICLES FACTORY, AVADI, CHENNAI 4. VARIANTS OF TANK a. BRIDGE LAYER TANK (BLT) HEAVY VEHICLES FACTORY, AVADI, CHENNAI VARIANTS OF TANK CONTINUED…. b. TRAWLS HEAVY VEHICLES FACTORY, AVADI, CHENNAI BUSINESS OPPORTUNITIES IN HVF There are huge opportunities for firms possessing process capabilities and expertise in • Fabrication /welding of pressed and machined components. • Manufacturing of pneumatic system operating at 150kgf/cm2 consisting of pneumatic valves. • Fabrication of pipelines of various sizes. • Manufacturing of dc motor , electromagnet with micro switches for armored fighting vehicles. • Mfg. of power, signal and data transmission cables of armored fighting vehicles. • Mfg. of electrical and electronics based control units for armored fighting vehicles. • Lamps/bulbs for armored fighting vehicles. • Mfg. Of rubber products like hoses, gaskets and seals etc. • Mfg. of castings, forgings and machined components and assemblies. HEAVY VEHICLES FACTORY, AVADI, CHENNAI CHALLENGES IN INDIGENISATION Constraints due to limited quantity. Non availability of raw material/inputs. Long lead time. Non availability of ToT. (Black box model) Non availability of design details. HEAVY VEHICLES FACTORY, AVADI, CHENNAI -

A Study on How the North Madras Films Are Portrayed in Tamil Cinema and Its Impact on Common Audience
International Journal of Research in Engineering, Science and Management 500 Volume-2, Issue-10, October-2019 www.ijresm.com | ISSN (Online): 2581-5792 A Study on how the North Madras Films are Portrayed in Tamil Cinema and its Impact on Common Audience J. John Felix Student, Department of Visual Communication, Loyola College, Chennai, India Abstract: The original home town of labours where they are settled in north madras (royaburam) during the Chennai floods accommodated the most. in the late 70s and 80s most of the places (2015) royaburam is one of the places in north madras which in north madras are slums. then government announced the slum was not affected by Chennai floods, there was no water logging clearance board act at the year 1971. After many years unemployment became a very rare condition because 9 out of 10 or stagnation, because of the well-constructed and executed people were employed and the education level has been drastically infrastructure of the area and also there was uninterrupted improved in the past 20 years. in Tamil cinema north madras and electricity, water & milk facility. this area is also home to one north madras peoples are portrayed in darker way like gangster, of the cities oldest railway stations. as the Chennai city uneducated, drug dealer. thus the film ends up to the audience that continues to expand its boundaries north madras continues to and makes them believe and assume that north madras it is the the place where the city began. same way shown in the film. the researcher in this study aims to find what is the audience impact on the films. -

Divine City Brochure Low
Development partner Site address: Shriram Divine City (Cybercity Mangadu Project Pvt Ltd) Kundranthur Main Road, Opp. Kamakshi Amman Temple, Mangadu, Chennai - 602101 Periya city. Office address: Shriram Properties Private Limited Lakshmi Neela Rite Choice Chamber, 1st floor, #9 Bazullah Road, T. Nagar, Chennai – 600 017 044 4001 4444 | www.shriramproperties.com/divinecity Niraya happiness. Disclaimer: *T&C apply. The information available on or through this advertisement/brochure is intended to provide general information and shall not be deemed to constitute any invitation, solicitation, offer or sale of any of our product offerings. The material is for customer’s reference, the company reserves the right to add, alter or delete material from the advertisement/brochure at any time. DEVELOPED BY CYBERCITY BUILDERS Mangadu gets it’s 2nd landmark A LOCALITY WITH A 1000 YEAR LEGACY NOW BECOMES YOUR HOME. Mangadu is a hidden jewel in West Porur visited by people from all over South India for its famous Amman temple. Strategically located off Mt. Poonamallee Road, just 10 mins from Outer Ring Road, it offers great connectivity to all parts of Chennai. Now the trusted brand Shriram Properties, part of the 90,000 Cr Shriram Group, brings you an incredible mini city, packed with conveniences and indulgences, all within the safety of a mega gated community. ’ Golden location. Great connectivity. COME, INVEST IN A LOCATION Multiple THAT TICKS conveniences. ALL THE BOXES Outer Ring Road MOGAPPAIR AVADI Road High Niraya Poonamallee connectivity MADURAVOYAL advantage Mt. Poonamallee Rd. Saveetha Kamakshi Medical College Amman Temple Road MainSHRIRAM JUST 5KM FROM PORUR, THIS AREA ENJOYS EASY CONNECTIVITY DIVINE CITY PORUR DLF IT TO AND MANGADU Park Kundrathur AND WILL SEE IN THE COMING YEARS. -

TAMBARAM MUNICIPALITY List of Hospitals S.No. Name of The
TAMBARAM MUNICIPALITY List of Hospitals S.No. Name of the Hospital 1. Deepam Hospital No. 327, Muthurangam Road, West Tambaram, Chennai - 45 2. Hindu Mission Hospital 39, GST Road, West Tambaram, Chennai - 45 3. Kasthuri Hospital 119, Shanmugam Road, West Tambaram, Chennai - 45 4. S.K. Nursing Home N0. 14, Duraiswamy Pillai Street, West Tambaram, Chennai - 45 5. V.N.Hospital No.1, Kattabomman Street, West Tambaram, Chennai - 45 6. K.K. Eye Care Hospital Gandhi Road, West Tambaram, Chennai - 45 7. Deepam Speciality Hospital No.24, M.K. Reddy Street, West Tambaram, Chennai - 45 8. Deepam Kidney Hospital GST Road, West Tambaram, Chennai 45 9. Dr. Agarwall’s Eye Hospital M.K. Reddy Street, West Tambaram, Chennai 45 10. N.N. Hospital 39, Gandhi Rod, West Tambaram, Chennai – 45. 11. Manoj Poly clinic Azhagesn Street, West Tambaram, Chennai - 45 12. Nirmal Eye Hospital Ayyasamy Street, West Tambaram, Chennai - 45 13. Sri Jeyam Hospital Ramakrishna Street, West Tambaram, Chennai - 45 14. N.S. Hospital Venkatesan Street, West Tambaram Chennai - 45 15. Rainbow Child Hospital VOC Street, West Tambaram, Chennai - 45 16. Doctor’s Plaza V.O.C. Street, West Tambaram, Chennai - 45 17. Sundar Health Centre, 10-A, Bakthavatchalam Street, West Tambaram, Chennai – 45. 18. Babu Maternity Hospital, 7, Duraisamy Reddy Street, West Tambaram, Chennai – 45. 19. Jawahar Hospital Lurtharan Church Street, West Tambaram 20 Chrisdudas Hospital No.1, Elangovan Street, East Tambaram, Chennai - 59 21. Bethesda Child Care Hospital 31, Bharathy Nagar, IOB Colony, East Tambaram 22. Cosh Hospital IAF Road, East Tambaram No.9, Duraisamy Nagar, Chennai - 56 23. -

Madhavaram Municipality
PROVIDING COMPREHENSIVE WATER SUPPLY SCHEME TO MADHAVARAM MUNICIPALITY The work of providing Comprehensive Water Supply Scheme to Madhavaram Municipality was taken up initially by CMWSSB as deposit work for Madhavaram Municipality at an estimated cost of Rs.55.00 crores. Administrative sanction was obtained from the Government for implementation of water supply scheme for the Madhavaram Municipality vide GO(D) No.85, MA& WS (MA2) Dept., Dated 21.02.2011 with Japan International Co-operation Agency funds. Madhavaram Municipality covered an extent of 17.41 sq.km and consists of about 607 streets for a length of about 150 Km. The comprehensive water supply scheme to Madhavaram Municipality has been designed to serve an ultimate population of about 3.83 lakhs expected for the ultimate year 2042 and the designed quantity of water supply is estimated to be about 59.43 MLD. The major components of proposed work include 1. Laying Distribution mains for a length of about 168.38 km. 2. Laying conveying mains for a length of about 4.30 km. 3. Construction of Headworks at six locations 4. Providing House Service Connections for 29,730 nos. Subsequently, the Government of Tamil Nadu vide GO(Ms) No.256, MA&WS (Election) Dept. dated 26.12.2009 had issued orders on expanding Chennai City by annexing 42 Adjacent local bodies and Madhavaram is one of the Municipalities in Chennai Metropolitan Area annexed with the Expanded Chennai city. As directed in the GO the administration of the expanded Chennai City came into effect from October 2011. Since then, it became the direct responsibility for the CMWSS Board to implement the water Supply scheme in the Madhavaram area. -

The Madras Torture Commission Report As a Liberal Response to a Crisis in Racial Capitalism
In Blood and Color: The Madras Torture Commission Report as a Liberal Response to a Crisis in Racial Capitalism By Aditya Kumar Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Bachelor Arts In the Department of History at Brown University Thesis Advisor: Naoko Shibusawa April 7, 2017 Table of Contents Acknowledgements 1 Introduction 2 Chapter One 11 Chapter Two 23 Chapter Three 52 Conclusion 73 Bibliography 79 Kumar 1 Acknowledgements My thanks to my family for providing me with unconditional love and support And to Professor Shibusawa for guiding me through every step of this project Kumar 2 Introduction To study Indian history, I faced the irony of traveling to the British Library in London. And while the walls of the library were lined with ornate paintings of Indian royalty, most of the academics conducting research were unmistakably white. Amidst this room of white academics, I rummaged through dozens of boxes containing trace files from different era of colonial rule. I initially set out to find Indian narratives, stories of Indians living under British rule and the institutions that shaped their experiences. Specifically, I planned on examining Indian experiences of police violence under British rule. Few documents in the archive, however, contained any reference to the lived experiences of Indians during British rule. Instead, I found myself limited by the constraints of a British archive. I encountered page after page of British perspectives, ranging from diary entries by British officers to extensive land surveys. Most folders contained incomplete records, or memos sloppily handwritten onto frail pieces of paper. -

Chennai District Origin of Chennai
DISTRICT PROFILE - 2017 CHENNAI DISTRICT ORIGIN OF CHENNAI Chennai, originally known as Madras Patnam, was located in the province of Tondaimandalam, an area lying between Pennar river of Nellore and the Pennar river of Cuddalore. The capital of the province was Kancheepuram.Tondaimandalam was ruled in the 2nd century A.D. by Tondaiman Ilam Tiraiyan, who was a representative of the Chola family at Kanchipuram. It is believed that Ilam Tiraiyan must have subdued Kurumbas, the original inhabitants of the region and established his rule over Tondaimandalam Chennai also known as Madras is the capital city of the Indian state of Tamil Nadu. Located on the Coromandel Coast off the Bay of Bengal, it is a major commercial, cultural, economic and educational center in South India. It is also known as the "Cultural Capital of South India" The area around Chennai had been part of successive South Indian kingdoms through centuries. The recorded history of the city began in the colonial times, specifically with the arrival of British East India Company and the establishment of Fort St. George in 1644. On Chennai's way to become a major naval port and presidency city by late eighteenth century. Following the independence of India, Chennai became the capital of Tamil Nadu and an important centre of regional politics that tended to bank on the Dravidian identity of the populace. According to the provisional results of 2011 census, the city had 4.68 million residents making it the sixth most populous city in India; the urban agglomeration, which comprises the city and its suburbs, was home to approximately 8.9 million, making it the fourth most populous metropolitan area in the country and 31st largest urban area in the world. -

Greater Chennai Corporation List of Public Information Officers & Appellate Authorities
Greater Chennai Corporation List of Public Information Officers & Appellate Authorities Sl. Subject Public Information Officer Appellate Authority No. 1 Establishment matter Deputy Collector (Admin), Assistant Commissioner(GA&P), related to General General Department, General Department, Department Greater Chennai Corporation, Greater Chennai Corporation, Amma Maligai, Amma Maligai, Ripon Building Campus, Ripon Building Campus, Chennai-600 003. Chennai-600 003. Ph: 044-25381815 / 25619202 Ph: 044-25383732 / 25619231 2 All subjects related to Accounts Officer, Assistant Commissioner (GA&P), Pension Section General Department(Pension), General Department, Greater Chennai Corporation, Greater Chennai Corporation, Amma Maligai, Amma Maligai, Ripon Building Campus, Ripon Building Campus, Chennai-600 003. Chennai-600 003. Ph: 044-25619295 Ph: 044-25383732 / 25619231 3 Buildings Executive Engineer, Superintending Engineer, Buildings Department, Buildings Department, Greater Chennai Corporation, Greater Chennai Corporation, Amma Maligai, Amma Maligai, Ripon Building Campus, Ripon Building Campus, Chennai-600 003. Chennai-600 003. Ph: 044-25619267 Ph: 044-25381580 / 25619212 4 Bridges Executive Engineer, Superintending Engineer, Bridges Department, Bridges Department, Greater Chennai Corporation, Greater Chennai Corporation, Amma Maligai, Amma Maligai, Ripon Building Campus, Ripon Building Campus, Chennai-600 003. Chennai-600 003. Ph: 044-25303667 / 668 / 669 Ph: 044-25381580 / 25619212 5 Solid Waste Executive Engineer, Superintending Engineer, -
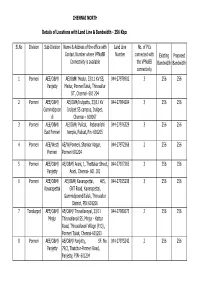
CHENNAI NORTH Sl.No Division Sub-Division Name & Address Of
CHENNAI NORTH Details of Locations with Land Line & Bandwidth - 256 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity 1 Ponneri AEE/O&M/ AE/O&M/ Medur, 33/11 KV SS, 044-27978902 3 256 256 Panjetty Medur, PonneriTaluk, Thiruvallur DT, Chennai- 601 204 2 Ponneri AEE/O&M/ AE/O&M/Irulipattu, 33/11 KV 044-27984204 3 256 256 Gummidipoon Irulipet SS campus, Irulipet, di Chennai – 600067 3 Ponneri AEE/O&M/ AE/O&M/ Pulicat, Pabanarishi 044-27976329 3 256 256 East Ponneri temple, Pulicat, Pin -601205 4 Ponneri AEE/West/ AE/W/Ponneri, Shankar Nagar, 044-27972368 2 256 256 Ponneri Ponneri 601204 5 Ponneri AEE/O&M/ AE/O&M/ Arani, 1, Thottakar Street, 044-27927265 3 256 256 Panjetty Arani, Chennai- 601 101 6 Ponneri AEE/O&M/ AE/O&M/ Kavarapettai, 465, 044-27925238 3 256 256 Kavarapettai GNT Road, Kavarapettai, GummidipoondiTaluk, Thiruvallur District, PIN 601206 7 Tondiarpet AEE/O&M/ AE/O&M/ Tiruvellavoyal, 33/11 044-27980675 2 256 256 Minjur Thiruvallavoil SS, Minjur - Kattur Road, Thiruvallavoil Village (P.O) , Ponneri Taluk, Chennai-601203 8 Ponneri AEE/O&M/ AE/O&M/ Panjetty, SF. No. 044-27975242 2 256 256 Panjetty 79/2, Thatchur-Ponneri Road, Panjetty, PIN- 601204 Details of Locations with Land Line & Bandwidth - 512 Kbps Sl.No Division Sub-Division Name & Address of the office with Land Line No. of PCs Contact Number where VPNoBB Number connected with Existing Proposed Connectivity is available the VPNoBB Bandwidth Bandwidth connectivity 1 T.Nagar Teynampet DMS SS, DMS Complex, Anna Salai, 044-24332950 3 512 512 Chennai – 6 2 T.Nagar Saidapet Thodunter Nagar SS, No.22, 044-24322211 3 512 512 Thodunter Nagar, Saidapet, CH- 15 3 T.Nagar Saidapet MHU SS, No.1 Link Road, MHU 044-24363191 1 512 512 Compound, CIT Nagar, Ch-35. -

Tamil Cinema
Centre for the Study of Communication and Culture Volume 28 (2009) No. 4 IN THIS ISSUE Tamil Cinema Perianayagam Jesudoss Salesian Pontifical University, Rome AQUARTERLY REVIEW OF COMMUNICATION RESEARCH ISSN: 0144-4646 Communication Research Trends Table of Contents Volume 28 (2009) Number 4 http://cscc.scu.edu Editor’s Introduction . 3 Published four times a year by the Centre for the Study of Tamil Cinema . 4 Communication and Culture (CSCC), sponsored by the 1. Introduction . 4 California Province of the Society of Jesus. A. Cinema as an aesthetic art . 4 Copyright 2009. ISSN 0144-4646 B. Indian cinema . 5 C. Cinema in Tamil Nadu . 5 Editor: William E. Biernatzki, S.J. 2. Origins of Tamil Cinema . 6 Managing Editor: Paul A. Soukup, S.J. A. Language . 6 B. Drama . 7 C. Music in Tamil drama . 7 Subscription: D. Loud voice culture in Tamil cinema . 8 Annual subscription (Vol. 28) US$50 3. History of Tamil Cinema . 8 A. Extent of Tamil cinema . 8 Payment by check, MasterCard, Visa or US$ preferred. B. A brief history of Tamil cinema . 9 For payments by MasterCard or Visa, send full account C. Technology and industry . 10 number, expiration date, name on account, and signature. D. Kollywood: Center of the Tamil cinema industry . 11 Checks and/or International Money Orders (drawn on 4. Film Distribution . 12 USA banks; for non-USA banks, add $10 for handling) 5. Cinema Production as Cultural Commodity should be made payable to Communication Research in Tamil Nadu . 13 Trends and sent to the managing editor 6. Consumption . 14 Paul A. -

Chennai North Commissionerate Jurisdiction
Chennai North Commissionerate Jurisdiction The jurisdiction of Chennai North Commissionerate will cover the areas covering Chennai Corporation Zone Nos. I to IX (From Ward Nos. I to 126 in existence as on OL-O4-2OL7) in the State of Tamil Nadu. The Continental shelf and exclusive economic zone contiguous to the eastern coast of India. No.26l1, Mahathma Gandhi Road, Nungambakkam, Location Chennai 600 O34 Divisions under the jurisdiction of Chennai North Commissionerate. Sl.No. Divisions 1, Thiruvottiyur Division 2. Madhavaram Division 3. Royapuram Division 4. Parrys Division 5. Egmore Division 6. Thiru Vi Ka Nagar Division 7. Ambattur Division 8. Anna Nagar Division 9. Pursawalkam Division 10. Nungambakkam Division 11. Triplicane Division L2. Mylapore Division Page 5 of 83 l.Thiruvottirnrr Division of Chennai North Commissionerate Location Ananda Complex, 459, Anna Salai, Te5rnampet, Chennai - 600 018 Jurisdiction Areas covering Ward Nos. 1 to L4 of Zone I & Ward Nos. 15 to 2Iof Zone II of Chennai Corporation, Taxpayers names starting with letters A, B, Y and Z of the Continental shelf and exclusive economic zone contiguous to the eastern course of India. The Division has five Ranges with jurisdiction as follows: Name of the Range Location Jurisdiction Areas covering ward Nos. L to 4 of Zone I, Taxpayers names starting with letters A of the Range I Continental shelf and exclusive econornic zone contiguous to the eastern course of India. Areas covering ward Nos. 5 to 9 of Zone I, Range II Taxpayers names starting with letters B of the Ananda Continental shelf and exclusive econornic zone Complex, contiguous to the eastern course of India.