Learning Objectives Chem
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Elimination Reactions E1, E2, E1cb and Ei
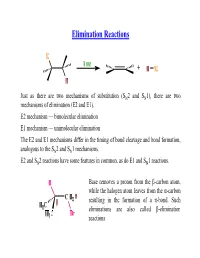
Elimination Reactions Dr. H. Ghosh Surendranath College, Kol-9 ________________________________________________ E1, E2, E1cB and Ei (pyrolytic syn eliminations); formation of alkenes and alkynes; mechanisms (with evidence), reactivity, regioselectivity (Saytzeff/Hofmann) and stereoselectivity; comparison between substitution and elimination. Substitution Reactions Elimination Reactions Elimination happens when the nucleophile attacks hydrogen instead of carbon Strong Base favor Elimination Bulky Nucleophile/Base favor Elimination High Temperature favors Elimination We know- This equation says that a reaction in which ΔS is positive is more thermodynamically favorable at higher temperature. Eliminations should therefore be favoured at high temperature Keep in Mind---- Mechanism Classification E1 Mechanism- Elimination Unimolecular E1 describes an elimination reaction (E) in which the rate-determining step is unimolecular (1) and does not involve the base. The leaving group leaves in this step, and the proton is removed in a separate second step E2 Mechanism- Elimination Bimolecular E2 describes an elimination (E) that has a bimolecular (2) rate-determining step that must involve the base. Loss of the leaving group is simultaneous with removal of the proton by the base Bulky t-butoxide—ideal for promoting E2 as it’s both bulky and a strong base (pKaH = 18). Other Organic Base used in Elimination Reaction These two bases are amidines—delocalization of one nitrogen’s lone pair on to the other, and the resulting stabilization of the protonated amidinium ion, E1 can occur only with substrates that can ionize to give relatively stable carbocations—tertiary, allylic or benzylic alkyl halides, for example. E1-Elimination Reaction not possible here The role of the leaving group Since the leaving group is involved in the rate-determining step of both E1 and E2, in general, any good leaving group will lead to a fast elimination. -
![[3+2]-ANNULATION REACTIONS with NITROALKENES in the SYNTHESIS of AROMATIC FIVE-MEMBERED NITROGEN HETEROCYCLES Vladimir A. Motorn](https://docslib.b-cdn.net/cover/7083/3-2-annulation-reactions-with-nitroalkenes-in-the-synthesis-of-aromatic-five-membered-nitrogen-heterocycles-vladimir-a-motorn-757083.webp)
[3+2]-ANNULATION REACTIONS with NITROALKENES in the SYNTHESIS of AROMATIC FIVE-MEMBERED NITROGEN HETEROCYCLES Vladimir A. Motorn
237 [3+2] - ANNULATION REACTIONS WIT H NITROALKENES IN THE SYNTHESIS OF AROMATIC FIVE - MEMBERED NITROGEN HETEROCYCLES DOI: http://dx.medra.org/ 10.17374/targets.2020.23.2 37 Vladimir A. Motornov, Sema L. Ioffe, Andrey A. Tabolin * N. D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky prosp. 47, 119991 Moscow, Russia (e - mail: [email protected]) Abstract. [3+2] - A nnulation reactions are widely used for the synthesis of aromatic heterocycles. In recent years they have become attractive for the preparation of medicinally relevant heterocycles due to their broad substrate scope and availability of starting materials . A nnulations with nitroalkenes may lead to different products due to the ability of the nitro - group to act both as activating group and as leaving group. Ultimately this gives rise to the synthesis of multifunctional heterocyclic compounds, including nitro - substituted ones. The present review covers various annulation re actions with nitroalkenes leading to five - membered nitrogen - containing heterocyclic rings. Oxidative annulation, annulation/elimination and self - oxidative annulation pathways are discussed. Contents 1. Introduction 2. Classification of nitroalkene - b ased annulation reactions 3. A nnulations with nitroalkenes in the synthesis of five - membered rings 3.1. Synthesis of pyrroles 3.1.1. Barton - Zard pyrrole synthesis 3.1.2. Annulation with enamines 3.1.3. Annulation with azomethine ylides 3.2. Synthesis of pyrazoles 3.2.1. Nitroalkene - diazo comp o unds [3+2] - cycloadditions 3.2.2. Oxidative annulation of nitroalkenes with hydrazones 3.3. Synthesis of imidazoles and imidazo[1,2 - a]pyridines 3.4. Synthesis of indolizines and related heter ocycles 3.5. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

Elimination Reactions Are Described
Introduction In this module, different types of elimination reactions are described. From a practical standpoint, elimination reactions widely used for the generation of double and triple bonds in compounds from a saturated precursor molecule. The presence of a good leaving group is a prerequisite in most elimination reactions. Traditional classification of elimination reactions, in terms of the molecularity of the reaction is employed. How the changes in the nature of the substrate as well as reaction conditions affect the mechanism of elimination are subsequently discussed. The stereochemical requirements for elimination in a given substrate and its consequence in the product stereochemistry is emphasized. ELIMINATION REACTIONS Objective and Outline beta-eliminations E1, E2 and E1cB mechanisms Stereochemical considerations of these reactions Examples of E1, E2 and E1cB reactions Alpha eliminations and generation of carbene I. Basics Elimination reactions involve the loss of fragments or groups from a molecule to generate multiple bonds. A generalized equation is shown below for 1,2-elimination wherein the X and Y from two adjacent carbon atoms are removed, elimination C C C C -XY X Y Three major types of elimination reactions are: α-elimination: two atoms or groups are removed from the same atom. It is also known as 1,1-elimination. H R R C X C + HX R Both H and X are removed from carbon atom here R Carbene β-elimination: loss of atoms or groups on adjacent atoms. It is also H H known as 1,2- elimination. R C C R R HC CH R X H γ-elimination: loss of atoms or groups from the 1st and 3rd positions as shown below. -

Elimination Reaction
Elimination reaction An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either one or two-step mechanism.[2] The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is Elimination reaction of cyclohexanol to bimolecular (second-order) while E1 is unimolecular (first-order). cyclohexene with sulfuric acid and In cases where the molecule is able to stabilize an anion but heat[1] possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism. Contents Loss of hydron (H+) E2 mechanism E1 mechanism Competition among mechanisms Elimination reactions other than β-elimination See also References External links Loss of hydron (H+) In most organic elimination reactions, at least one hydron (H+) is lost to form the double bond: the unsaturation of the molecule increases. It is also possible that a molecule undergoes reductive elimination, by which the valence of an atom in the molecule decreases by two, though this is more common in inorganic chemistry. An important class of elimination reactions is those involving alkyl halides, with good leaving groups, reacting with a Lewis base to form an alkene. Elimination may be considered the reverse of an addition reaction. When the substrate is asymmetric, regioselectivity is determined by Zaitsev's rule or through Hofmann elimination if the carbon with the most substituted hydrogen is inaccessible. -

Elimination Reactions
Elimination Reactions Elimination reactions discussed here are reactions that produce alkenes, usually from the loss of two particles, x-y, from a substrate. The nucleophilic substitution reactions discussed in another section are often accompanied by elimination reactions as competing reactions. Conditions can be met that make the elimination reaction the main reaction. Fluorine can be eliminated as fluoride but it is the least reactive of the halogens because of its strong C-F bond. Thus for halogens the rate of elimination is I>Br>Cl>F. Fluorine, because of its great electronegativity, can cause the beta hydrogen to become acidic. The mechanism of elimination form many fluorinated compounds involves a carbanion in the E1cb (Elimination first order carbanion) mechanism. Stereoselectiviy of elimination is not always observed. Elimination first order (E1) Secondary substrates give products from both substitution and elimination, by first rate limiting ionization of the substrate to produce a carbocation. The carbocation then can react with a nucleophile (SN1) or lose a beta hydrogen (E1) to form an alkene. Of course, carbocations can do other things such as rearrange to a more stable carbocation followed by substitution and elimination. The E1 mechanism is unlikely for fluorinated compounds because of the requirement to break the strong carbon-fluorine bond in the rate determining step. CH3 H Ph OCH3 Racemic mixture SN1 CH3 CH3OH H H CH3 Ph I Ph E1 R enantiomer a carbocation H CH2 Ph The elimination reactions requires removal of a beta hydrogen by a base. Thus increasing the base strength of the reaction medium increases the amount of elimination product. -

Reaction-Mechanisms-GOC-Book.Pdf
158 Essential Notes on General Organic Chemistry (G.O.C.) CHAPTER Reaction Mechanisms 4 Topics Coverage :- • Substitution Reactions • Free Radical Reactions • SN1 Mechanism • SN2 Mechanism • SN1 v/s SN2 Mechanisms • E1 Mechanism • E2 Mechanism • E1 v/s E2 Mechanisms • Substitution v/s Elimination Reactions • Aromatic Substitution Electrophilic Mechanism • Aromatic Substitution Nucleophilic Mechanism • Addition Nucleophilic Mechanism • Addition Electrophilic Mechanism • Addition Free Radical Mechanism • RARE MECHANISMS : (SNi, E1cb, SN', NGP, Benzyne, Ipso) • Rearrangements Introduction : How exactly does an organic reaction take place is the subject of study in this chapter of Reaction Mechanisms. Just as detectives investigate a crime after it has taken place and arrive at a reasonable prediction about how the crime may have occurred, similarly organic chemists predict by a number of laboratory techniques using sophisticated instrumentation how an organic reaction may have taken place. Many of these techniques are based on the Physical Chemistry principles of Redox, Equilibria, Chemical Kinetics and Thermodynamics. The instruments used include Polarimeter, NMR Spectroscope, Electric Dipole measurement device, isotopic tracer, pH meter etc. CLASSIFICATION OF ORGANIC REACTIONS Organic Reactions in JEE syllabi can be classified as under : 1. Substitution reactions - (i) Free radical (ii) Nucleophilic-SN1, SN2,SNi (iii) Electrophilic 2. Elimination reactions-E1, E2 & E1cb 3. Addition reactions - (i) Electrophilic (ii) Nucleophilic (iii) Free Radical 4. Rearrangements The IITian’s Prashikshan Kendra Pvt. Ltd. Essential Notes on General Organic Chemistry (G.O.C.) 159 1. SUBSTITUTION REACTIONS A reaction in which one group or atom is replaced by another is called a substitution reaction. The incoming group is bonded to the same carbon to which the leaving group was bonded. -
Elimination Reactions
Elimination Reactions Just as there are two mechanisms of substitution (SN2 and SN1), there are two mechanisms of elimination (E2 and E1). E2 mechanism — bimolecular elimination E1 mechanism — unimolecular elimination The E2 and E1 mechanisms differ in the timing of bond cleavage and bond formation, analogous to the SN2and SN1 mechanisms. E2 and SN2 reactions have some features in common, as do E1 and SN1 reactions. Base removes a proton from the β-carbon atom, while the halogen atom leaves from the α-carbon resulting in the formation of a π-bond. Such eliminations are also called β-elimination reactions The Zaitsev (Saytseff) Rule When alkyl halides have two or more different β carbons, more than one alkene product is formed. In such cases, the major product is the more stable product—the one with the more substituted double bond. This phenomenon is called the Zaitsev rule. The Zaitsev product or the more substituted alkene product is more stable than the less substituted product. The stability of the more substituted alkene is a result of number of different contributing factors, including hyperconjugation. Each alkyl group that can involve in hyperconjugation with the double bond stabilizes it by approximately 6 kcal/mol The E2 Mechanism The most common mechanism for dehydrohalogenation is the E2 mechanism. It exhibits second-order kinetics, and both the alkyl halide and the base appear in the rate equation − rate = k[(CH3)3CBr][HO ] The reaction is concerted—all bonds are broken and formed in a single step. E2 reactions are regioselective and favor the formation of Zaitsev products. -

(SN2) Reaction
Alkyl Halides Substitution and Elimination 1 Nomenclature • Look for the longest chain that contains the maximum number of functional groups, in this case the halogen is the functional group and so even though the cyclohexane has more carbon atoms, the main chain is the two carbon ethane chain, the structure is named as a substituted alkyl bromide Br H * 2 1 (1R)-bromo-1-cyclohexylethane named as a substiuted alkyl halide therefore, cyclohexane is a substituent! 2 Second Order Nucleophilic Substitution (SN2) Reaction Substitution by making a new bond at the same time as breaking the old bond • Substitution requires a bond to be broken and a new bond to be formed • The LOWEST energy way of doing this (unless precluded by steric or other effects, see later) is to make the new bond (getting some energy "back") at the same time as breaking the old bond, this is SN2 Example: CONCERTED sp2 ‡ H H H LB (R) (S) nucleophile H–O * HO C Br C* C Et + Br Et Br HO Me Et Me Me LA leaving group "backside attack!" electrophile partial bonds • Reactions in which all bonds are made and broken at the same time are called CONCERTED • This is fundamentally just a Lewis acid/base reaction of a kind we have seen previously, the Lewis base has the high energy chemically reactive electrons, which are used to make a new bond to the Lewis acid, and a stronger bond is formed (C-O in the example above) and a weaker bond is broken (C-Br above) • HO– is the Lewis Base and Nucleophile • the halide is the Lewis acid/electrophile • the Br– anion is the Leaving Group • This reaction "goes" because…. -

Elimination and Substitution Reactions
University of Tikrit Department of Chemistry College of Science Elimination and Substitution Reactions Lecture in Organic Chemistry By Doctoral students Anwar Latif Khalil And Hind Fawzi Arif University of Tikrit Department of Chemistry College of Science Elimination Reactions – E1 Reaction: The E1 Mechanism The E1 reaction proceeds via a two-step mechanism: the bond to the leaving group breaks first before the π bond is formed. The slow step is unimolecular, involving only the alkyl halide. It exhibits first-order kinetics, rate = k[(CH3)3CCl] E1 reactions also are regioselective and follow Zaitsev rule University of Tikrit Department of Chemistry College of Science Energy Profile for an E1 Reaction Factors Affecting the Rate of an E1 Reaction The rate of an E1 reaction increases as the number of R groups on the carbon with the leaving group increases. The strength of the base usually determines whether a reaction follows the E1 or E2 mechanism. Strong bases like OH− and OR− favor E2 reactions, whereas weaker bases like H2O and ROH favor E1 reactions. Characteristics of an E1 Reaction University of Tikrit Department of Chemistry College of Science Kinetics – First order Mechanism – Two steps Identity of R group – More substituted halides react faster Rate: R3CX > R2CHX > RCH2X Strength of the base – Favored by weaker bases such as H2O and ROH Leaving group – Better leaving group leads to faster reaction rates. Just as in SN1 reactions, the rate determining step involves the C—X bond cleavage Type of solvent – Favored by polar protic solvents, which can stabilize the ionic intermediates The Zaitsev (Saytseff) Rule When alkyl halides have two or more different β carbons, more than one alkene product is formed. -

Density Functional Theory Study of the Mechanisms and Stereochemistry
Published on Web 12/17/2010 Density Functional Theory Study of the Mechanisms and Stereochemistry of the Rh(I)-Catalyzed Intramolecular [3+2] Cycloadditions of 1-Ene- and 1-Yne-Vinylcyclopropanes Lei Jiao, Mu Lin, and Zhi-Xiang Yu* Beijing National Laboratory for Molecular Sciences (BNLMS), Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry, Peking UniVersity, Beijing 100871, China Received August 25, 2010; E-mail: [email protected] Abstract: The mechanisms, structures of all stationary points involved, and kinetic and thermody- namic parameters of the Rh(I)-catalyzed intramolecular [3+2] cycloaddition reactions of 1-ene- and 1-yne-vinylcyclopropanes (1-ene-VCPs and 1-yne-VCPs) have been investigated using density functional theory (DFT) calculations. The computational results showed that the [3+2] reactions of 1-ene/yne-VCPs studied here occur through a catalytic cycle of substrate-catalyst complex formation, cyclopropane cleavage, alkene/alkyne insertion, and reductive elimination. Alkene/alkyne insertion is the rate- and stereoselectivity- determining step of these multistep [3+2] cycloadditions. The experimentally observed high reactivity of 1-yne-VCPs compared to 1-ene-VCPs is well rationalized by the differences of steric effects in the alkyne/ alkene insertion transition states. DFT calculations unveiled that the relative orientation of the tethers in the 1-ene/yne-VCPs plays a key role in controlling the stereochemistry of the [3+2] cycloadducts. In addition, DFT calculation results are used to explain why, in some cases, the formation of the -hydride elimination byproduct can compete with the [3+2] pathway. -

Chapter 9 Formation of Alkenes and Alkynes
(2/94)(5-8/96)(7,8/01)(1,2/02)(10-12/03) Neuman Chapter 9 Chapter 9 Formation of Alkenes and Alkynes. Elimination Reactions from Organic Chemistry by Robert C. Neuman, Jr. Professor of Chemistry, emeritus University of California, Riverside [email protected] <http://web.chem.ucsb.edu/~neuman/orgchembyneuman/> Chapter Outline of the Book ************************************************************************************** I. Foundations 1. Organic Molecules and Chemical Bonding 2. Alkanes and Cycloalkanes 3. Haloalkanes, Alcohols, Ethers, and Amines 4. Stereochemistry 5. Organic Spectrometry II. Reactions, Mechanisms, Multiple Bonds 6. Organic Reactions *(Not yet Posted) 7. Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic Substitution 8. Alkenes and Alkynes 9. Formation of Alkenes and Alkynes. Elimination Reactions 10. Alkenes and Alkynes. Addition Reactions 11. Free Radical Addition and Substitution Reactions III. Conjugation, Electronic Effects, Carbonyl Groups 12. Conjugated and Aromatic Molecules 13. Carbonyl Compounds. Ketones, Aldehydes, and Carboxylic Acids 14. Substituent Effects 15. Carbonyl Compounds. Esters, Amides, and Related Molecules IV. Carbonyl and Pericyclic Reactions and Mechanisms 16. Carbonyl Compounds. Addition and Substitution Reactions 17. Oxidation and Reduction Reactions 18. Reactions of Enolate Ions and Enols 19. Cyclization and Pericyclic Reactions *(Not yet Posted) V. Bioorganic Compounds 20. Carbohydrates 21. Lipids 22. Peptides, Proteins, and α−Amino Acids 23. Nucleic Acids ************************************************************************************** *Note: Chapters marked with an (*) are not yet posted. 0 (2/94)(5-8/96)(7,8/01)(1,2/02)(10-12/03) Neuman Chapter 9 9: Formation of Alkenes and Alkynes. Elimination Reactions Preview 9-3 9.1 Elimination Reactions 9-3 Common Features of Elimination Reactions (9.1A) 9-3 General Equations.