Spatial Patterns and Drivers of Angiosperm Sexual Systems in China Differ Between Woody and Herbaceous Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Sex Ratio and Spatial Distribution of Male and Female Antennaria Dioica
Sex ratio and spatial distribution of male and female Antennaria dioica (Asteraceae) plants Sandra Varga* and Minna-Maarit Kytöviita Department of Biological and Environmental Science, University of Jyväskylä, P.O. Box 35, FIN- 40014 Jyväskylä, Finland E-mail addresses: [email protected] and [email protected] (S. Varga), minna- [email protected] (M.-M. Kytöviita) *Author for correspondence: Sandra Varga Department of Biological and Environmental Science University of Jyväskylä FIN-40014 Jyväskylä Finland Email: [email protected] and [email protected] Phone: +358 14 260 2304 Fax: +358 14 260 2321 1 Abstract Sex ratio, sex spatial distribution and sexual dimorphism in reproduction and arbuscular mycorrhizal colonisation were investigated in the dioecious clonal plant Antennaria dioica (Asteraceae). Plants were monitored for five consecutive years in six study plots in Oulanka, northern Finland. Sex ratio, spatial distribution of sexes, flowering frequency, number of floral shoots and the number and weight of inflorescences were recorded. In addition, intensity of mycorrhizal fungi in the roots was assessed. Both sexes flowered each year with a similar frequency, but the overall genet sex ratio was strongly female-biased. The bivariate Ripley’s analysis of the sex distribution showed that within most plots sexes were randomly distributed except for one plot. Sexual dimorphism was expressed as larger floral and inflorescence production and heavier inflorescences in males. In addition, the roots of both sexes were colonised to a similar extent by arbuscular mycorrhizal fungi. The female sex-biased flowering ratios reported are not consistent among years and cannot be explained in terms of spatial segregation of the sexes or sex lability. -

Literature Cited
Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volumes 19, 20, and 21, whether as selected references, in text, or in nomenclatural contexts. In citations of articles, both here and in the taxonomic treatments, and also in nomenclatural citations, the titles of serials are rendered in the forms recommended in G. D. R. Bridson and E. R. Smith (1991). When those forms are abbre- viated, as most are, cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. In nomenclatural citations (only), book titles are rendered in the abbreviated forms recommended in F. A. Stafleu and R. S. Cowan (1976–1988) and F. A. Stafleu and E. A. Mennega (1992+). Here, those abbreviated forms are indicated parenthetically following the full citations of the corresponding works, and cross references to the full citations are interpolated in the list alphabetically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix “a”; thus, the sequence of explicit suffixes begins with “b”. Works missing from any suffixed sequence here are ones cited elsewhere in the Flora that are not pertinent in these volumes. -

Field Release of the Hoverfly Cheilosia Urbana (Diptera: Syrphidae)
USDA iiillllllllll United States Department of Field release of the hoverfly Agriculture Cheilosia urbana (Diptera: Marketing and Regulatory Syrphidae) for biological Programs control of invasive Pilosella species hawkweeds (Asteraceae) in the contiguous United States. Environmental Assessment, July 2019 Field release of the hoverfly Cheilosia urbana (Diptera: Syrphidae) for biological control of invasive Pilosella species hawkweeds (Asteraceae) in the contiguous United States. Environmental Assessment, July 2019 Agency Contact: Colin D. Stewart, Assistant Director Pests, Pathogens, and Biocontrol Permits Plant Protection and Quarantine Animal and Plant Health Inspection Service U.S. Department of Agriculture 4700 River Rd., Unit 133 Riverdale, MD 20737 Non-Discrimination Policy The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual's income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) To File an Employment Complaint If you wish to file an employment complaint, you must contact your agency's EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. Additional information can be found online at http://www.ascr.usda.gov/complaint_filing_file.html. To File a Program Complaint If you wish to file a Civil Rights program complaint of discrimination, complete the USDA Program Discrimination Complaint Form (PDF), found online at http://www.ascr.usda.gov/complaint_filing_cust.html, or at any USDA office, or call (866) 632-9992 to request the form. -

Alkemade.Pdf
Lisse, October 2019 Gebr. Th. & W. Alkemade B.V. Rooversbroekdijk 121 2161 LP LISSE THE NETHERLANDS Telnr. 0031 (0) 252 - 22 33 98 Faxnr.0031 (0) 252 - 22 34 09 E-mail: [email protected] Website: www.gebr-alkemade.nl Dear customer, Enclosed you will find our new catalogue for 2020 with a complete assortment of our young plants in trays. All trays are pre-treated by a machine before delivery which enables you to take the plants out easier. You will save time, and the risk of damaging them is being reduced. Marked varieties: W: Youngplants from roots: These plants are made only once in a certain amount. Mainly deliverable after +/- week 20 and have to be ordered as early as possible; K: Normal cuttings: no remarks. T.C.: Tissue Culture G: Grasses BSTC: Cuttings produced from tissue-culture stockplants and grown in clean boxes for one year. Majority of the plugs is provided with a growcoon and delivered in a 102 plugtray. However because of unforeseen circumstances we may not be able to use a growcoon and/or a different tray We hope you can find your selection in our catalogue. Should you be interested in plants, not mentioned in this catalogue, please contact us. We will be happy to make you an offer without any obligation on your part. You are welcome to visit our nursery at any time and make sure of the quality of our plants. We also want to inform you that we can send the catalogue by e-mail. If you want to receive it by e-mail, please let us know. -

Jan Scholten Wonderful Plants Leseprobe Wonderful Plants Von Jan Scholten Herausgeber: Alonnissos Verlag
Jan Scholten Wonderful Plants Leseprobe Wonderful Plants von Jan Scholten Herausgeber: Alonnissos Verlag http://www.narayana-verlag.de/b14446 Im Narayana Webshop finden Sie alle deutschen und englischen Bücher zu Homöopathie, Alternativmedizin und gesunder Lebensweise. Das Kopieren der Leseproben ist nicht gestattet. Narayana Verlag GmbH, Blumenplatz 2, D-79400 Kandern Tel. +49 7626 9749 700 Email [email protected] http://www.narayana-verlag.de 0.1.4 Foreword Lou Klein Hahnemann, trained as a medical translator, researcher and chemist, was at the forefront of science as it was known in his time. In the beginning of homeopathy’s introduction, he led a fervor of pioneering activity and introduced many substances as homeopathic remedies. These were carefully identified and classified as best they could be by the standards of the time, as Hahnemann was a stickler for careful methodologies. Many of his students and followers, such as Hering and Kent, went on to prolifically introduce remedies and clinical concepts in order to advance homeopathy. But as an allopathic “scientific method” took over medicine at the beginning of the 20th century, homeopathy’s growth and momentum lagged. Relative to the time that passed and the developments in science and medicine, minimal evolution and progress in the homeopathic profession was made. There were many reasons for this, notwithstanding the attack on homeopathy from without by allopaths claiming their territory and from within homeopathy where a anachronistic conservative even dogmatically religious ethic took over. Few new homeopathic remedies or techniques were introduced into homeopathy and old systems of classification were relied upon to define and relate what small number of remedies had already been introduced and used. -
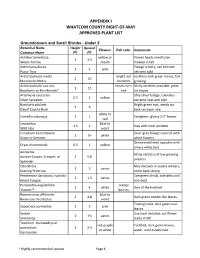
Whatcom County's Approved Plant List
APPENDIX I WHATCOM COUNTY RIGHT-OF-WAY APPROVED PLANT LIST Groundcovers and Small Shrubs - Under 2' Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Achillea tomentosa yellow or Flower heads need to be 1 3-5 Wooly Yarrow cream mowed in fall Antennaria dioica Foliage is furry, can tolerate 1 2 pink Pussy Toes extreme cold Arctostaphylos media bright red Leathery dark green leaves, fast 2 10 Manzanita Media branches growing Arctostaphylos uva ursi leaves turn Many varieties available, great 1 15 Bearberry or Kinnikinnick* red for slopes Artemesia caucasica Silky silver foliage, tolerates 0.5 2 yellow Silver Spreader extreme heat and cold Baccharis pilularis Bright green mat, needs cut 2 6 Dwarf Coyote Bush back once per year white to Camellia sasanqua 1 1 Evergreen, glossy 2-3" leaves red Ceanothus blue to 1.5 2 Stay with local varieties Wild Lilac violet Cerastium tomentosum Silver gray foliage covered with 1 5+ white Snow-in-Summer white flowers Ornamental seed capsules with Dryas drummondii 0.5 1 yellow silvery white tails Juniperus Many varieties of low growing Juniper-Carpet, Creeper, or 2 5-8 junipers Spreader Oenothera May die back in severe winters, 1 2 varies Evening Primrose come back strong Penstemon davidsonii, rupicola Evergreen shrub, tolerates cold 2 1.5 varies Beard Tongue and heat Pyracantha augustifolia orange 1 4 white One of the hardiest 'Gnome'* berries Rosmariunus officinalis blue to 2 4-8 Dark green needle-like leaves Rosemary 'Prostratus' violet Trailing habit, dark green oval Saponaria ocymoides 1 3 pink leaves Sedum Use local varieties, cut flower 2 1-5 varies Stonecrop stalks in fall Teucrium chamaedrys or red-purple Toothed, dark green leaves, postratium 1 2-3 or white water until established Germander * Highly-recommended species Page 1 Thymus white to 2 1-2 Many varieties Thyme purple Shrubs Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Arctostaphylos bright red Shiny dark green leaves turn 2-15 20 varies Manzanita berries maroon in fall. -

Distributions of Vascular Plants in the Czech Republic. Part 2
Preslia 88: 229–322, 2016 229 Distributions of vascular plants in the Czech Republic. Part 2 Rozšíření cévnatých rostlin v České republice. Část 2 Zdeněk K a p l a n1,JiříDanihelka1, 2,JitkaŠtěpánková1, Libor E k r t3, Jindřich C h r t e k Jr.1,JiříZázvorka1,VítGrulich2, Radomír Ř e p k a4, Jan P r a n č l1, 5,MichalDucháček6,PavelKúr3, Kateřina Š u m b e r o v á1 &JosefBrůna1 1Institute of Botany, The Czech Academy of Sciences, CZ-252 43 Průhonice, Czech Repub- lic, e-mail: [email protected], [email protected], [email protected], zazvorka @ibot.cas.cz, [email protected], [email protected], [email protected], [email protected]; 2Department of Botany and Zoology, Masaryk University, Kotlářská 2, CZ-611 37 Brno, Czech Republic, e-mail: [email protected], [email protected]; 3 Department of Botany, Faculty of Science, University of South Bohemia, Branišovská 1760, CZ-370 05 České Budějovice, Czech Republic, email: [email protected], [email protected]; 4Department of Forest Botany, Dendrology and Geobiocenology, Faculty of Forestry and Wood Technology, Mendel University, Zemědělská 3, CZ-613 00 Brno, Czech Republic, e-mail: [email protected]; 5Department of Botany, Faculty of Science, Charles University in Prague, Benátská 2, CZ-128 01 Prague, Czech Republic; 6Department of Botany, National Museum, Cirkusová 1740, CZ-193 00 Praha 9-Horní Počernice, Czech Republic, e-mail: [email protected] Kaplan Z., Danihelka J., Štěpánková J., Ekrt L., Chrtek J. Jr., Zázvorka J., Grulich V., Řepka R., Prančl J., Ducháček M., Kúr P., Šumberová K. -

Plants Unlimited Pussytoes
[email protected] 207.594.7754 P.O. Box 374 629 Commercial St. Rockport, Maine 04856 Pussytoes* Antennaria dioica Height: 6 inches Spread: 12 inches Sunlight: Hardiness Zone: 3a Other Names: Pussy Toes, Pussy-Toes Description: A low growing selection perfect for groundcover, borders or garden beds; clusters of silvery-white flowers sit atop grey-green foliage; a drought tolerant variety that requires little to no maintenance Ornamental Features Pussytoes in bloom Photo courtesy of NetPS Plant Finder Pussytoes's attractive tiny tomentose narrow leaves remain grayish green in color with hints of silver throughout the year. It has balls of white flowers at the ends of the stems from late spring to early summer, which emerge from distinctive silver flower buds, and which are interesting on close inspection. The fruit is not ornamentally significant. Landscape Attributes Pussytoes is an herbaceous evergreen perennial with an upright spreading habit of growth. It brings an extremely fine and delicate texture to the garden composition and should be used to full effect. This is a relatively low maintenance plant, and should not require much pruning, except when necessary, such as to remove dieback. Deer don't particularly care for this plant and will usually leave it alone in favor of tastier treats. It has no significant negative characteristics. Pussytoes is recommended for the following landscape applications; - Mass Planting - Rock/Alpine Gardens - Border Edging - General Garden Use - Groundcover - Naturalizing And Woodland Gardens Visit plants-unlimited.com [email protected] 207.594.7754 P.O. Box 374 629 Commercial St. Rockport, Maine 04856 Planting & Growing Pussytoes will grow to be only 6 inches tall at maturity, with a spread of 12 inches. -

SPECIES IDENTIFICATION GUIDE National Plant Monitoring Scheme SPECIES IDENTIFICATION GUIDE
National Plant Monitoring Scheme SPECIES IDENTIFICATION GUIDE National Plant Monitoring Scheme SPECIES IDENTIFICATION GUIDE Contents White / Cream ................................ 2 Grasses ...................................... 130 Yellow ..........................................33 Rushes ....................................... 138 Red .............................................63 Sedges ....................................... 140 Pink ............................................66 Shrubs / Trees .............................. 148 Blue / Purple .................................83 Wood-rushes ................................ 154 Green / Brown ............................. 106 Indexes Aquatics ..................................... 118 Common name ............................. 155 Clubmosses ................................. 124 Scientific name ............................. 160 Ferns / Horsetails .......................... 125 Appendix .................................... 165 Key Traffic light system WF symbol R A G Species with the symbol G are For those recording at the generally easier to identify; Wildflower Level only. species with the symbol A may be harder to identify and additional information is provided, particularly on illustrations, to support you. Those with the symbol R may be confused with other species. In this instance distinguishing features are provided. Introduction This guide has been produced to help you identify the plants we would like you to record for the National Plant Monitoring Scheme. There is an index at -

Sex Dimorphism in Shoot Morphology of Antennaria Dioica (L.) Gaertner (Compositae)
Botanica Pacifica. A journal of plant science and conservation. 2019. 8(1): 51–56 DOI: 10.17581/bp.2019.08105 Sex dimorphism in shoot morphology of Antennaria dioica (L.) Gaertner (Compositae) Ksenia V. Dudova Ksenia V. Dudova ABSTRACT e-mail: [email protected] Determination the sex of the plant shoots without using features of a separate ca pi tulum is important in population-based studies. I tested hypothesis that Anten Department of Geobotany, Faculty of naria dioica has statistically significant differences in morphological traits between Biology, Lomonosov Moscow State Uni- female and male shoots. Eight morphological features and 1907 shoots from the versity, Moscow, Russia three geographically separated regions were studied. Reliable difference was found in height of generative shoots, rosette diameter and number of cauline leaves. However, the traits studied had significant overlap within individual populations and did not demonstrate significant differencces within a region. Therefore, none Manuscript received: 22.01.2016 of the traits presented can be used to determine the sex of an individual. Review completed: 10.01.2019 Keywords: dioecy, sex dimorphism, Antennaria dioica, morphological traits Accepted for publication: 28.01.2019 Published online: 31.01.2019 РЕЗЮМЕ Дудова К.В. Проявления полового диморфизма в морфологии по- бегов Antennaria dioica (L.) Gaertner (Compositae). В популяционных исследованиях двудомных растений актуально определение пола побегов без использования признаков генеративной сферы. Для Antennaria dioica проверена гипотеза о том, что по морфологическим признакам женские и мужские побеги этого вида значимо различаются. Для исследования по- лового ди морфизма выбрано восемь признаков. Изучено 1907 побегов из трех гео гра фически удаленных регионов. -

Norwegian Journal of Entomology
Norwegian Journal of Entomology Volume 50 No. 1 • 2003 Published by the Norwegian Entomological Society Oslo and Stavanger NORWEGIAN JOURNAL OF ENTOMOLOGY A continuation of Fauna Norvegica Serie B (1979-1998), Norwegian Journal of Entomology (1975-1978) and Norsk entomologisk Tidsskrift (1921-1974). Published by The Norwegian Entomological Society (Norsk ento mologisk forening). Norwegian Journal of Entomology publishes original papers and reviews on taxonomy, faunistics, zoogeography, general and applied ecology of insects and related terrestrial arthropods. Short communications, e.g. one or two printed pages, are also considered. Manuscripts should be sent to the editor. Editor Lauritz Semme, Department of Biology, University of Oslo, P.o.Box 1050 Blindern, N-0316 Oslo, Norway. E mail: [email protected]. Editorial secretary Lars Ove Hansen, Zoological Museum, University of Oslo, PO.Box 1172, Blindern, N-0318 Oslo. E-mail: [email protected]. Editorial board Arne C. Nilssen, Tromse John O. Solem, Trondheim Lita Greve J ensen, Bergen Knut Rognes, Stavanger Arne Fjellberg, Tjeme Membership and subscription. Requests about membership should be sent to the secretary: Jan A. Stenlekk, PO. Box 386, NO-4002 Stavanger, Norway [email protected]).Annual membership fees for The Norwegian Ento mological Society are as follows: NOK 200 Uuniors NOK 100) for members with addresses in Norway, NOK 250 for members in Denmark, Finland and Sweden, NOK 300 for members outside Fennoscandia and Denmark. Members of The Norwegian Entomological Society receive Norwegian Journal of Entomology and Insekt-Nytt free. Institutional and non-member subscription: NOK 250 in Fennoscandia and Denmark, NOK 300 elsewhere. -

Santa Fe Botanical Garden Museum Hill Garden Plant List
SANTA FE BOTANICAL GARDEN MUSEUM HILL GARDEN PLANT LIST WELCOME GARDEN TREES Juniperus scopulorum ‘Moonglow’ Moonglow Juniper Koelreuteria paniculata Golden Raintree Malus x 'Indian Magic' Indian Magic Crabapple Quercus macrocarpa Bur Oak SHRUBS Agave havardiana Havard’s Agave Agave parryi Parry’s Agave Caryopteris x clandonensis ‘Dark Knight’ Dark Knight Blue Mist Chamaebatiaria millefolium Fernbush Yucca rostrata Beaked Yucca VINES Campsis x tagliabuana ‘Madame Galen’ Madame Galen Trumpet Vine Lonicera japonica ‘Halliana’ Hall’s Honeysuckle GRASSES Muhlenbergia reverchonii ‘Autumn Embers' Autumn Embers Muhly PERENNIALS Agastache cana 'Sinning' Sonoran Sunset Agastache Salvia pachyphylla Mojave Sage GROUNDCOVERS Thymus neiceffii Juniper Leaf Thyme Thymus serpyllum 'Pink Chintz' Pink Chintz Thyme Thymus serpyllum 'Reiter' Reiter Thyme Veronica pectinata Wooly Veronica page 1 of 10 SANTA FE BOTANICAL GARDEN MUSEUM HILL GARDEN PLANT LIST MEADOW GARDEN TREES Cupressus arizonica ‘Blue Ice’ Blue Ice Arizona Cypress Juniperus scopulorum ‘Medora’ Medora Juniper Juniperus scopulorum ‘Moonglow’ Moonglow Juniper Malus x 'Indian Magic' Indian Magic Crabapple Malus x ‘Radiant’ Radiant Crabapple Quercus muehlenbergii Chinkapin Oak Ulmus propinqua ‘JFS-Bieberich’ Emerald Sunshine Elm SHRUBS Ephedra equisetina Bluestem Joint Fir Prunus besseyi Western Sand Cherry Rhus trilobata Threeleaf Sumac PERENNIALS Agastache rupestris Sunset Agastache Calylophus hartwegii Sundrops Linum perenne lewisii Blue Flax Penstemon barbatus Scarlet Bugler Penstemon barbatus x mexicali ‘Pike’s Peak Purple’ Pike’s Peak Penstemon Penstemon barbatus x mexicali ‘Red Rocks' Red Rocks Penstemon Penstemon rostriflorus Bridges' Penstemon Sphaeralcea munroana Munro's Globemallow GRASSES Eragrostis trichodes Sand Lovegrass page 2 of 10 SANTA FE BOTANICAL GARDEN MUSEUM HILL GARDEN PLANT LIST GROUNDCOVERS Sedum kamtschaticum Kamschatka Sedum Sedum rupestre ‘Angelina’ Angelina Sedum Sempervivum spp.