1 Sex Ratio and Spatial Distribution of Male and Female Antennaria Dioica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Literature Cited
Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volumes 19, 20, and 21, whether as selected references, in text, or in nomenclatural contexts. In citations of articles, both here and in the taxonomic treatments, and also in nomenclatural citations, the titles of serials are rendered in the forms recommended in G. D. R. Bridson and E. R. Smith (1991). When those forms are abbre- viated, as most are, cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. In nomenclatural citations (only), book titles are rendered in the abbreviated forms recommended in F. A. Stafleu and R. S. Cowan (1976–1988) and F. A. Stafleu and E. A. Mennega (1992+). Here, those abbreviated forms are indicated parenthetically following the full citations of the corresponding works, and cross references to the full citations are interpolated in the list alphabetically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix “a”; thus, the sequence of explicit suffixes begins with “b”. Works missing from any suffixed sequence here are ones cited elsewhere in the Flora that are not pertinent in these volumes. -

Fdn22 Northern Dry-Bedrock Pine (Oak) Woodland *Spinulose Shield Fern Or Glandular Wood Fern ( Dryopteris Carthusiana Or D
FIRE-DEPENDENT FOREST/WOODLAND SYSTEM FDn22 Northern Floristic Region Northern Dry-Bedrock Pine (Oak) Woodland Dry pine or oak woodlands on shallow, excessively drained, loamy soils on bedrock ridges and hillsides or on rock ledges and terraces adjacent to rivers. Crown and surface fires were common historically. Vegetation Structure & Composition Description is based on summary of vegetation data from 47 plots (relevés). Ground-layer cover of forbs and grami- noids typically ranges from 25-75%. The most common vascular plants are Canada mayflower (Maianthemum canadense), wild sarsaparilla (Aralia nudicaulis), large-leaved aster (Aster macrophyllus), poverty grass (Danthonia spicata), wintergreen (Gaultheria procumbens), and bracken (Pteridium aqui- linum). Lichen- and moss-covered bedrock and boulders typically make up at least 25% of the ground layer. Shrub layer is typically dominated by deciduous species, usually with patchy to interrupted cover (25-75%). Lowbush blue- berry (Vaccinium angustifolium), juneberries (Amelanchier spp.), red maple saplings, and bush honeysuckle (Diervilla lonicera) are the most common species in the shrub layer. Subcanopy is usually absent, but when present, red maple and paper birch are frequent components. Canopy is composed of conifers, hardwoods, or conifers mixed with hardwoods, and is usually patchy (25-50% cover), with openings in areas of exposed bedrock or boulders. Red pine and white pine are dominant on many sites. On other sites, jack pine or northern pin oak are dominant. In mixed forests, conifers often form a supercanopy above hardwood species. Paper birch is often present in the hardwood canopy. Landscape Setting & Soils Glacially scoured bedrock—Common. Landscape is hummocky to rugged. Parent material is non-calcareous drift, usually less than 20in (50cm) deep over bedrock. -

Prairie Restoration Technical Guides
Optimal Collection Period Seed Ripening Period EARLY SEASON NATIVE FORBS May June July August September EARLY SEASON NATIVE FORBS May June July August SCIENTIFIC NAME COMMON NAME 1-10 10-20 20-30 1-10 10-20 20-30 1-10 10-20 20-30 1-10 10-20 20-30 1-10 10-20 20-30 SCIENTIFIC NAME COMMON NAME 1-10 10-20 20-30 1-10 10-20 20-30 1-10 10-20 20-30 1-10 10-20 20-30 Caltha palustris Marsh marigold LATE SEASON NATIVE FORBS August September October November SEED COLLECTING Prairie smoke SCIENTIFIC NAME COMMON NAME 1-10 10-20 20-30 1-10 10-20 20-30 1-10 10-20 20-30 1-10 10-20 20-30 FROM Antennaria neglecta Pussytoes Stachys palustris Woundwort Castilleja coccinea Indian paintbrush Vicia americana Vetch False dandelion Rudbeckia hirta Black-eyed Susan TALLGRASS PRAIRIES Saxifraga pensylvanica Swamp saxifrage Lobelia spicata Spiked lobelia Senecio aureus Golden ragwort Iris shrevei Sisyrinchium campestre Blue-eyed grass Hypoxis hirsuta Yellow star grass Rosa carolina Pasture rose Content by Greg Houseal Pedicularis canadensis Lousewort Oxypolis rigidior Cowbane PRAIRIE RESTORATION SERIES V Prairie violet Vernonia fasciculata Ironweed Cardamine bulbosa Spring cress Veronicastrum virginicum Culver's root Allium canadense Wild garlic Heliopsis helianthoides Seed of many native species are now Lithospermum canescens Hoary puccoon L Narrow-leaved loosestrife commercially1 available for prairie Phlox maculata Marsh phlox Lythrum alatum Winged loosestrife Phlox pilosa Prairie phlox reconstructions, large or small. Yet many Ceanothus americana New Jersey tea Anemone canadensis Canada anemone Eupatorium maculatum Spotted Joe Pye people have an interest in collecting Prunella vulgaris var. -

The Vascular Flora of Rarău Massif (Eastern Carpathians, Romania). Note Ii
Memoirs of the Scientific Sections of the Romanian Academy Tome XXXVI, 2013 BIOLOGY THE VASCULAR FLORA OF RARĂU MASSIF (EASTERN CARPATHIANS, ROMANIA). NOTE II ADRIAN OPREA1 and CULIŢĂ SÎRBU2 1 “Anastasie Fătu” Botanical Garden, Str. Dumbrava Roşie, nr. 7-9, 700522–Iaşi, Romania 2 University of Agricultural Sciences and Veterinary Medicine Iaşi, Faculty of Agriculture, Str. Mihail Sadoveanu, nr. 3, 700490–Iaşi, Romania Corresponding author: [email protected] This second part of the paper about the vascular flora of Rarău Massif listed approximately half of the whole number of the species registered by the authors in their field trips or already included in literature on the same area. Other taxa have been added to the initial list of plants, so that, the total number of taxa registered by the authors in Rarău Massif amount to 1443 taxa (1133 species and 310 subspecies, varieties and forms). There was signaled out the alien taxa on the surveyed area (18 species) and those dubious presence of some taxa for the same area (17 species). Also, there were listed all the vascular plants, protected by various laws or regulations, both internal or international, existing in Rarău (i.e. 189 taxa). Finally, there has been assessed the degree of wild flora conservation, using several indicators introduced in literature by Nowak, as they are: conservation indicator (C), threat conservation indicator) (CK), sozophytisation indicator (W), and conservation effectiveness indicator (E). Key words: Vascular flora, Rarău Massif, Romania, conservation indicators. 1. INTRODUCTION A comprehensive analysis of Rarău flora, in terms of plant diversity, taxonomic structure, biological, ecological and phytogeographic characteristics, as well as in terms of the richness in endemics, relict or threatened plant species was published in our previous note (see Oprea & Sîrbu 2012). -

Field Release of the Hoverfly Cheilosia Urbana (Diptera: Syrphidae)
USDA iiillllllllll United States Department of Field release of the hoverfly Agriculture Cheilosia urbana (Diptera: Marketing and Regulatory Syrphidae) for biological Programs control of invasive Pilosella species hawkweeds (Asteraceae) in the contiguous United States. Environmental Assessment, July 2019 Field release of the hoverfly Cheilosia urbana (Diptera: Syrphidae) for biological control of invasive Pilosella species hawkweeds (Asteraceae) in the contiguous United States. Environmental Assessment, July 2019 Agency Contact: Colin D. Stewart, Assistant Director Pests, Pathogens, and Biocontrol Permits Plant Protection and Quarantine Animal and Plant Health Inspection Service U.S. Department of Agriculture 4700 River Rd., Unit 133 Riverdale, MD 20737 Non-Discrimination Policy The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual's income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) To File an Employment Complaint If you wish to file an employment complaint, you must contact your agency's EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. Additional information can be found online at http://www.ascr.usda.gov/complaint_filing_file.html. To File a Program Complaint If you wish to file a Civil Rights program complaint of discrimination, complete the USDA Program Discrimination Complaint Form (PDF), found online at http://www.ascr.usda.gov/complaint_filing_cust.html, or at any USDA office, or call (866) 632-9992 to request the form. -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Alkemade.Pdf
Lisse, October 2019 Gebr. Th. & W. Alkemade B.V. Rooversbroekdijk 121 2161 LP LISSE THE NETHERLANDS Telnr. 0031 (0) 252 - 22 33 98 Faxnr.0031 (0) 252 - 22 34 09 E-mail: [email protected] Website: www.gebr-alkemade.nl Dear customer, Enclosed you will find our new catalogue for 2020 with a complete assortment of our young plants in trays. All trays are pre-treated by a machine before delivery which enables you to take the plants out easier. You will save time, and the risk of damaging them is being reduced. Marked varieties: W: Youngplants from roots: These plants are made only once in a certain amount. Mainly deliverable after +/- week 20 and have to be ordered as early as possible; K: Normal cuttings: no remarks. T.C.: Tissue Culture G: Grasses BSTC: Cuttings produced from tissue-culture stockplants and grown in clean boxes for one year. Majority of the plugs is provided with a growcoon and delivered in a 102 plugtray. However because of unforeseen circumstances we may not be able to use a growcoon and/or a different tray We hope you can find your selection in our catalogue. Should you be interested in plants, not mentioned in this catalogue, please contact us. We will be happy to make you an offer without any obligation on your part. You are welcome to visit our nursery at any time and make sure of the quality of our plants. We also want to inform you that we can send the catalogue by e-mail. If you want to receive it by e-mail, please let us know. -

Jan Scholten Wonderful Plants Leseprobe Wonderful Plants Von Jan Scholten Herausgeber: Alonnissos Verlag
Jan Scholten Wonderful Plants Leseprobe Wonderful Plants von Jan Scholten Herausgeber: Alonnissos Verlag http://www.narayana-verlag.de/b14446 Im Narayana Webshop finden Sie alle deutschen und englischen Bücher zu Homöopathie, Alternativmedizin und gesunder Lebensweise. Das Kopieren der Leseproben ist nicht gestattet. Narayana Verlag GmbH, Blumenplatz 2, D-79400 Kandern Tel. +49 7626 9749 700 Email [email protected] http://www.narayana-verlag.de 0.1.4 Foreword Lou Klein Hahnemann, trained as a medical translator, researcher and chemist, was at the forefront of science as it was known in his time. In the beginning of homeopathy’s introduction, he led a fervor of pioneering activity and introduced many substances as homeopathic remedies. These were carefully identified and classified as best they could be by the standards of the time, as Hahnemann was a stickler for careful methodologies. Many of his students and followers, such as Hering and Kent, went on to prolifically introduce remedies and clinical concepts in order to advance homeopathy. But as an allopathic “scientific method” took over medicine at the beginning of the 20th century, homeopathy’s growth and momentum lagged. Relative to the time that passed and the developments in science and medicine, minimal evolution and progress in the homeopathic profession was made. There were many reasons for this, notwithstanding the attack on homeopathy from without by allopaths claiming their territory and from within homeopathy where a anachronistic conservative even dogmatically religious ethic took over. Few new homeopathic remedies or techniques were introduced into homeopathy and old systems of classification were relied upon to define and relate what small number of remedies had already been introduced and used. -
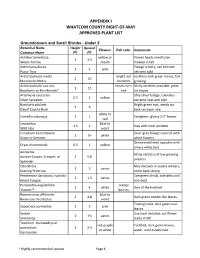
Whatcom County's Approved Plant List
APPENDIX I WHATCOM COUNTY RIGHT-OF-WAY APPROVED PLANT LIST Groundcovers and Small Shrubs - Under 2' Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Achillea tomentosa yellow or Flower heads need to be 1 3-5 Wooly Yarrow cream mowed in fall Antennaria dioica Foliage is furry, can tolerate 1 2 pink Pussy Toes extreme cold Arctostaphylos media bright red Leathery dark green leaves, fast 2 10 Manzanita Media branches growing Arctostaphylos uva ursi leaves turn Many varieties available, great 1 15 Bearberry or Kinnikinnick* red for slopes Artemesia caucasica Silky silver foliage, tolerates 0.5 2 yellow Silver Spreader extreme heat and cold Baccharis pilularis Bright green mat, needs cut 2 6 Dwarf Coyote Bush back once per year white to Camellia sasanqua 1 1 Evergreen, glossy 2-3" leaves red Ceanothus blue to 1.5 2 Stay with local varieties Wild Lilac violet Cerastium tomentosum Silver gray foliage covered with 1 5+ white Snow-in-Summer white flowers Ornamental seed capsules with Dryas drummondii 0.5 1 yellow silvery white tails Juniperus Many varieties of low growing Juniper-Carpet, Creeper, or 2 5-8 junipers Spreader Oenothera May die back in severe winters, 1 2 varies Evening Primrose come back strong Penstemon davidsonii, rupicola Evergreen shrub, tolerates cold 2 1.5 varies Beard Tongue and heat Pyracantha augustifolia orange 1 4 white One of the hardiest 'Gnome'* berries Rosmariunus officinalis blue to 2 4-8 Dark green needle-like leaves Rosemary 'Prostratus' violet Trailing habit, dark green oval Saponaria ocymoides 1 3 pink leaves Sedum Use local varieties, cut flower 2 1-5 varies Stonecrop stalks in fall Teucrium chamaedrys or red-purple Toothed, dark green leaves, postratium 1 2-3 or white water until established Germander * Highly-recommended species Page 1 Thymus white to 2 1-2 Many varieties Thyme purple Shrubs Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Arctostaphylos bright red Shiny dark green leaves turn 2-15 20 varies Manzanita berries maroon in fall. -

Distributions of Vascular Plants in the Czech Republic. Part 2
Preslia 88: 229–322, 2016 229 Distributions of vascular plants in the Czech Republic. Part 2 Rozšíření cévnatých rostlin v České republice. Část 2 Zdeněk K a p l a n1,JiříDanihelka1, 2,JitkaŠtěpánková1, Libor E k r t3, Jindřich C h r t e k Jr.1,JiříZázvorka1,VítGrulich2, Radomír Ř e p k a4, Jan P r a n č l1, 5,MichalDucháček6,PavelKúr3, Kateřina Š u m b e r o v á1 &JosefBrůna1 1Institute of Botany, The Czech Academy of Sciences, CZ-252 43 Průhonice, Czech Repub- lic, e-mail: [email protected], [email protected], [email protected], zazvorka @ibot.cas.cz, [email protected], [email protected], [email protected], [email protected]; 2Department of Botany and Zoology, Masaryk University, Kotlářská 2, CZ-611 37 Brno, Czech Republic, e-mail: [email protected], [email protected]; 3 Department of Botany, Faculty of Science, University of South Bohemia, Branišovská 1760, CZ-370 05 České Budějovice, Czech Republic, email: [email protected], [email protected]; 4Department of Forest Botany, Dendrology and Geobiocenology, Faculty of Forestry and Wood Technology, Mendel University, Zemědělská 3, CZ-613 00 Brno, Czech Republic, e-mail: [email protected]; 5Department of Botany, Faculty of Science, Charles University in Prague, Benátská 2, CZ-128 01 Prague, Czech Republic; 6Department of Botany, National Museum, Cirkusová 1740, CZ-193 00 Praha 9-Horní Počernice, Czech Republic, e-mail: [email protected] Kaplan Z., Danihelka J., Štěpánková J., Ekrt L., Chrtek J. Jr., Zázvorka J., Grulich V., Řepka R., Prančl J., Ducháček M., Kúr P., Šumberová K. -

Antennaria Corymbosa Plant
Antennaria corymbosa Plant Meadow pussy-toes Flowering/Fruiting: June to A mat-forming perennial. August Leaves mostly basal, narrow, hairy. Kathy Ahlenslager Look Alikes: Antennaria rosea. Antennaria corymbosa has narrower leaves. Bracts 1/8 to 1/4 Habitat in. high, green Bogs. and Elevation 5000 ft. hairy at base. Bract tips have a blackish or dark brown spot below white terminal portion. University of Washington Press. Illustration by Jeanne R. Janish. Kathy Ahlenslager Antennaria parvifolia Plant Nutall’s pussy-toes Flowering/Fruiting: May to July. A mat forming perennial seldom over 6 in. tall. Dry flower bracts bright white to dull white to Photo by BLM pink, 1/4 to 1/3 in. long. Look Alikes: Antennaria rosea. Antennaria parvifolia is stouter with closely bunched flowers and has longer leaves. Bracts 1/4 to 1/2 in. high. Habitat Dry open areas, on sandy or Basal leaves wooly with hairs of medium gravelly riverbanks, openings length, oblong of Ponderosa pine forests. but narrowed at Elevation 1900 to 2600 ft.. base, 1/2 to 1 1/2 in. long and 1/4 to 1/2 in. wide. Photo by BLM University of Washington Press. Illustration by Jeanne R. Janish. Astragalus microcystis Plant Least bladdery milk-vetch Flowering/Fruiting: Late April through August. A tap-rooted perennial with many stems arising from an exposed rootcrown. Amy Cabral Flowers pink or pale lavender to deep magenta purplish, Look Alikes: Astragalus canadensis var. mortonii and A. alpinus var. alpinus. Astralagus microcystis is shorter Leaves 9 to 15 5 to 12 stalked. grayish-green, than A. -

Plants Unlimited Pussytoes
[email protected] 207.594.7754 P.O. Box 374 629 Commercial St. Rockport, Maine 04856 Pussytoes* Antennaria dioica Height: 6 inches Spread: 12 inches Sunlight: Hardiness Zone: 3a Other Names: Pussy Toes, Pussy-Toes Description: A low growing selection perfect for groundcover, borders or garden beds; clusters of silvery-white flowers sit atop grey-green foliage; a drought tolerant variety that requires little to no maintenance Ornamental Features Pussytoes in bloom Photo courtesy of NetPS Plant Finder Pussytoes's attractive tiny tomentose narrow leaves remain grayish green in color with hints of silver throughout the year. It has balls of white flowers at the ends of the stems from late spring to early summer, which emerge from distinctive silver flower buds, and which are interesting on close inspection. The fruit is not ornamentally significant. Landscape Attributes Pussytoes is an herbaceous evergreen perennial with an upright spreading habit of growth. It brings an extremely fine and delicate texture to the garden composition and should be used to full effect. This is a relatively low maintenance plant, and should not require much pruning, except when necessary, such as to remove dieback. Deer don't particularly care for this plant and will usually leave it alone in favor of tastier treats. It has no significant negative characteristics. Pussytoes is recommended for the following landscape applications; - Mass Planting - Rock/Alpine Gardens - Border Edging - General Garden Use - Groundcover - Naturalizing And Woodland Gardens Visit plants-unlimited.com [email protected] 207.594.7754 P.O. Box 374 629 Commercial St. Rockport, Maine 04856 Planting & Growing Pussytoes will grow to be only 6 inches tall at maturity, with a spread of 12 inches.