Hairstyling Module 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Manchus: a Horse of a Different Color
History in the Making Volume 8 Article 7 January 2015 Manchus: A Horse of a Different Color Hannah Knight CSUSB Follow this and additional works at: https://scholarworks.lib.csusb.edu/history-in-the-making Part of the Asian History Commons Recommended Citation Knight, Hannah (2015) "Manchus: A Horse of a Different Color," History in the Making: Vol. 8 , Article 7. Available at: https://scholarworks.lib.csusb.edu/history-in-the-making/vol8/iss1/7 This Article is brought to you for free and open access by the History at CSUSB ScholarWorks. It has been accepted for inclusion in History in the Making by an authorized editor of CSUSB ScholarWorks. For more information, please contact [email protected]. Manchus: A Horse of a Different Color by Hannah Knight Abstract: The question of identity has been one of the biggest questions addressed to humanity. Whether in terms of a country, a group or an individual, the exact definition is almost as difficult to answer as to what constitutes a group. The Manchus, an ethnic group in China, also faced this dilemma. It was an issue that lasted throughout their entire time as rulers of the Qing Dynasty (1644- 1911) and thereafter. Though the guidelines and group characteristics changed throughout that period one aspect remained clear: they did not sinicize with the Chinese Culture. At the beginning of their rule, the Manchus implemented changes that would transform the appearance of China, bringing it closer to the identity that the world recognizes today. In the course of examining three time periods, 1644, 1911, and the 1930’s, this paper looks at the significant events of the period, the changing aspects, and the Manchus and the Qing Imperial Court’s relations with their greater Han Chinese subjects. -
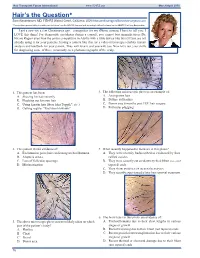
Hair's the Question*
Hair Transplant Forum International www.ISHRS.org March/April 2015 Hair’s the Question* Sara Wasserbauer, MD, FISHRS Walnut Creek, California, USA [email protected] *The questions presented by the author are not taken from the ABHRS item pool and accordingly will not be found on the ABHRS Certifying Examination. I got a new toy a few Christmases ago—a magnifier for my iPhone camera. I have to tell you: I LOVE this thing! For diagnositic usefulness during a consult, you cannot beat magnification (Dr. Nicole Rogers even won the poster competition in Alaska with a little device like this)! If you are not already using it for your patients, having a camera like this (or a video microscope) enables instant analysis and feedback for your patient. They will love it and you will, too. Now let’s test your skills for diagnosing some of these commonly seen photomicrographs of the scalp. 1. This patient has been: 4. The following microscopic photo is an example of: A. Shaving his hair recently A. An ingrown hair B. Plucking out his own hair B. Diffuse folliculitis C. Using keratin hair fibers (aka Toppik®, etc.) C. Donor area 6 months post FUE hair surgery D. Getting regular “Brazilian blowouts” D. Follicular plugging 2. This patient shows evidence of: 5. What recently happened to the hairs in this photo? A. Exclamation point hairs indicating trichotillomania A. They were recently backcombed as evidenced by their B. Alopecia areata ruffled cuticle. C. Loss of follicular openings B. They were recently cut as shown by their blunt (i.e., not D. -

Hairdressing: Fashion Updo
Hairdressing: Fashion Updo Learning outcomes Maintain effective and safe methods of working when creating an up-do hairstyle with suitable products, tools, equipment and accessories. Create a balanced, fashion up-do suitable for an individual client and demonstrate professional, creative skills. Introduction Tools required for this treatment Wedding Fashion Gown Put up/ back brushing brush The Look: Pin tail comb This look is a modern fashion look currently popular with Slim traditional cutting comb brides or bridesmaids who are looking for a romantic 4/5 long, slim sectioning clips without catches to prevent ’boho’ feel for an outdoor/ country garden / natural marking the hair theme. The look includes a lace braid. An assortment of Kirby grips to suit Adapt this style for prom looks by using different client’s hair colour accessories. Approx. 20 straight long, fine grips to fix and separate Accessories: Hair spray- firm hold/ Shine Spray Works well with flowers, ivy or comb pins accessories. Smoothing crème Oil / or serum Alternatively, use no accessories for casual day wear. Heat protector Tongs or styling irons/ straighteners If required, wadding or added hair depending on hair thickness Accessories (flowers, veil) Step 1 Part the hair diagonally as a small zig-zag approx. 9cm from the front hair line. Take into account a preferred parting (ideally side to soften the look) and the client’s head shape. Use mirror to pull down tendrils around the face, avoiding symmetry. Then create a circular section from the top of the head point to the ears. Clip out of the way. Tip: 1. -

The End of the Queue: Hair As Symbol in Chinese History Michael Godley
East Asian History NUMBER 8 . DECEMBER 1994 THE CONTINUATION OF Paperson Far EasternHistory Institute of Advanced Studies Australian National University Editor Geremie R. Barme Assistant Editor Helen Lo Editorial Board John Clark Mark Elvin (Convenor) Helen Hardacre John Fincher Andrew Fraser Colin Jeffcott W. J. F. Jenner Lo Hui-min Gavan McCormack David Marr Tessa Morris-Suzuki Michael Underdown Business Manager Marion Weeks Production Helen Lo Design Maureen MacKenzie (Em Squared Typographic Design), Helen Lo Printed by Goanna Print, Fyshwick, ACT This is the eighth issue of East Asian History in the series previously entitled Papers on Far Eastern History. The journal is published twice a year. Contributions to The Editor, East Asian History Division of Pacific & Asian History, Research School of Pacific & Asian Studies Australian National University, Canberra ACT 0200, Australia Phone +61 62493140 Fax +61 62495525 Subscription Enquiries Subscription Manager, East Asian History, at the above address Annual Subscription Australia A$45 Overseas US$45 (for two issues) iii CONTENTS 1 Mid-Ch'ing New Text (Chin-wen) Classical Learning and its Han Provenance: the Dynamics of a Tradition of Ideas On-cho Ng 33 From Myth to Reality: Chinese Courtesans in Late-Qing Shanghai Christian Henriot 53 The End of the Queue: Hair as Symbol in Chinese History Michael Godley 73 Broken Journey: Nhfti Linh's "Going to France" Greg and Monique Lockhart 135 Chinese Masculinity: Theorising' Wen' and' Wu ' Kam Louie and Louise Edwards iv Cover calligraphy Yan Zhenqing �JU!iUruJ, Tang calligrapher and statesman Cover picture The walled city of Shanghai (Shanghai xianzhi, 1872) THE END OF THE QUEUE: HAIR AS SYMBOL IN CHINESE HISTORY ..J1! Michael R. -

Catalogo-2021-EN.Pdf
We are Beardburys, sexy, cool, nonconformist, we like to innovate and go beyond. We are the grooming leading brand for bad guy s, trusted by you, your barber & hairdresser. essence Alternative Conventionalism bores us! We like to be transcendent, Sexy to break the rules, to seek Show our most sensual side adventures, to discover new is very common on us. routes... We enjoy life and the great We love to surprise with the pleasures that come with unexpected, we are original it... we leave the door open and to the imagination . We like to open paths but above all, leave a mark. Daring The answer to any craziness is and will be always: YES! Rogue We are playful and we like to We cannot live without adrenaline joke. we enjoy every second to the Naughtiness runs in our veins fullest. as gas pass through the injectors. We like to take risks and there is nothing that makes us more uncomfor- table than feeling like being Genuine just one of the bunch. Natural and authentic as life itself. Every time we start to create we think out of the box, design and innovation come always with us. The essence of our formulas, the scents, the textures, the packa- ging, the colours, the small details... ¡ We challenge the traditional male grooming market ! PRODUCTS CATEGORIES WAXES AND POMADES FIXATION TREATMENT 4 5-7 8-9 BEARD AND SHAVE COLORATION TOOLS & FURNITURE 10-11 12 13-16 DESINFECTION & ACCESORIES EXHIBITORS & DISPOSABLES 18-20 MERCHANDISING 17 21-27 30ml/100ml 30ml/100ml 30ml/100ml Natural Pomade Matte Pomade Matt-Clay Pomade The ideal pomade for styling your hair and giving it Authentic barber shop wax with a matte finish. -

(BBL™) / LASER HAIR REMOVAL Pre- and Post-Care Instructions
Broad Band Light (BBL™) / LASER HAIR REMOVAL Pre- and Post-Care Instructions Please read the following carefully, as this information will prepare you for BBL / Laser Hair Removal, what to expect, and how to care for the treated area. PRE - LASER INSTRUCTIONS 1. Shave the site/s to be treated the evening before each treatment session. (Avoid waxing for 2 weeks before and throughout treatment course – as waxing diminishes laser target.) 2. Also, minimize sun exposure, as best as possible, for at least 1 month before and after treatment. Wear protective clothing (hat, etc.) and a high SPF (at least SPF 30) sunblock to protect the treated area/s from direct sun exposure. 3. Also, do not use aspirin, aspirin containing medications or alcohol for at least 1 week before and for the first 2 days after treatment. Take Tylenol or another pain reliever which contains no aspirin or ibuprofen, if needed. 4. Apply EMLA / ELA-Max / Topicaine (or other topical anesthetic) at least 1 hour before treatment under saran wrap, if needed. WHAT TO EXPECT ♦ BBL/Laser-treated hairs will still be visible / present after your treatment. However, they are now injured or destroyed at the level of the hair follicle (“root”) and will soon fall out (usually, within the next 2-5 days up to 2 weeks). ♦ The treated area may redden and/or swell somewhat. A mild sunburn sensation may be noticed for up to several hours after treatment. This is caused by the BBL/laser energy, and represents inflammation, and not infection. It is normal and expected part of healing process. -

A Randomized, Doubleblind, Placebo and Activecontrolled, Halfhead
BJD CONCISE COMMUNICATION British Journal of Dermatology A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata A. Trink,1 E. Sorbellini,1 P. Bezzola,1 L. Rodella,2 R. Rezzani,2 Y. Ramot3 and F. Rinaldi1 1International Hair Research Foundation (IHRF), Milan, Italy 2University of Brescia, Brescia, Italy 3Department of Dermatology, Hadassah – Hebrew University Medical Center, Jerusalem, Israel Summary Correspondence Background Alopecia areata (AA) is a common autoimmune condition, causing Fabio Rinaldi. inflammation-induced hair loss. This disease has very limited treatment possibili- E-mail: [email protected] ties, and no treatment is either curative or preventive. Platelet-rich plasma (PRP) has emerged as a new treatment modality in dermatology, and preliminary evi- Accepted for publication 16 April 2013 dence has suggested that it might have a beneficial role in hair growth. Objectives To evaluate the efficacy and safety of PRP for the treatment of AA in a Funding sources randomized, double-blind, placebo- and active-controlled, half-head, parallel- None. group study. Methods Forty-five patients with AA were randomized to receive intralesional Conflicts of interest injections of PRP, triamcinolone acetonide (TrA) or placebo on one half of None declared. their scalp. The other half was not treated. Three treatments were given for each DOI 10.1111/bjd.12397 patient, with intervals of 1 month. The endpoints were hair regrowth, hair dystrophy as measured by dermoscopy, burning or itching sensation, and cell proliferation as measured by Ki-67 evaluation. Patients were followed for 1 year. -

COSMETOLOGY CURRICULUM | Styling
COSMETOLOGY CURRICULUM | Styling Book ONE | Student Guide COSMETOLOGY CURRICULUM | Styling TABLE OF CONTENTS 1. Introduction to Styling Hair..................................................................3 2. Finger Waving Technique........................................................................11 3. Curl Bases and Stems................................................................................18 4. Pin Curls (Flat and Volume)....................................................................22 5. Roller Setting and Curl Variations........................................................34 6. Back-Combing and Back-Brushing.......................................................40 7. Half-Round Brush Air Forming Technique........................................45 8. Round Brush Styling Technique............................................................51 9. Finger Drying and Palm Drying............................................................57 10. Thermal Techniques for Curling...........................................................62 11. Thermal Techniques for Creating Waves.........................................73 12. Thermal Techniques for Smoothing and Straightening.............81 13. French Twist................................................................................................91 14. Draped Style...............................................................................................97 15. Chignon........................................................................................................101 -

Treasures of Darkness
BAT IN A WHIRLWIND By Richard Balthazar Chapter 2. TREASURES OF DARKNESS TREASURES OF DARKNESS # Today being a Tuesday and a school day, I had to get up at six. Washing up in the bathroom didn’t take long because I didn’t shave yet, though I’d been hairy since about thirteen and had wonderful fuzz growing on my face. Folks liked my blue eyes and long lashes. I wore my brown hair in a flattop, real plain because Daddy said ducktail looked sissy. I didn’t really like the hairdo, but it looked a lot better than a crew-cut. Daddy also said I had a skinny chicken neck and ears like a cab with both doors hanging open. I had to admit my ears did stick out a bit, but I’d squash them back whenever I thought to. Still, I considered myself fairly handsome, hopefully enough for Annette to fall in love with me. Sister Janie left a bit later for her grade school in Lockesburg, the town about six miles north that we kids called Lockjaw. On my way back through her room, I shook her mattress to rouse her, and she snarled like an irritated cat. Another shake got her to sit up with her dark hair all awry, so I considered my fraternal duty accomplished. Over at the café, Melba brought me out some soft-scrambled eggs and sausage and as always waited for me to say they were good. Naturally Daddy told me to hurry up. He always wanted to leave just a few minutes before I got done with the funny papers. -

Hair: the Performance of Rebellion in American Musical Theatre of the 1960S’
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Winchester Research Repository University of Winchester ‘Hair: The Performance of Rebellion in American Musical Theatre of the 1960s’ Sarah Elisabeth Browne ORCID: 0000-0003-2002-9794 Doctor of Philosophy December 2017 This Thesis has been completed as a requirement for a postgraduate research degree of the University of Winchester MPhil/PhD THESES OPEN ACCESS / EMBARGO AGREEMENT FORM This Agreement should be completed, signed and bound with the hard copy of the thesis and also included in the e-copy. (see Thesis Presentation Guidelines for details). Access Permissions and Transfer of Non-Exclusive Rights By giving permission you understand that your thesis will be accessible to a wide variety of people and institutions – including automated agents – via the World Wide Web and that an electronic copy of your thesis may also be included in the British Library Electronic Theses On-line System (EThOS). Once the Work is deposited, a citation to the Work will always remain visible. Removal of the Work can be made after discussion with the University of Winchester’s Research Repository, who shall make best efforts to ensure removal of the Work from any third party with whom the University of Winchester’s Research Repository has an agreement. Agreement: I understand that the thesis listed on this form will be deposited in the University of Winchester’s Research Repository, and by giving permission to the University of Winchester to make my thesis publically available I agree that the: • University of Winchester’s Research Repository administrators or any third party with whom the University of Winchester’s Research Repository has an agreement to do so may, without changing content, translate the Work to any medium or format for the purpose of future preservation and accessibility. -

Policy 312 PROFESSIONAL APPEARANCE STANDARDS Page 2 of 4
Policy 312 Subject PROFESSIONAL APPEARANCE STANDARDS Date Published Page 26 August 2017 1 of 4 By Order of the Police Commissioner POLICY 1. Personal Discipline, Public Confidence and Respect. These Professional Appearance Standards are established to encourage public confidence and respect, to instill a degree of personal discipline among members, to facilitate easy recognition of officers in the field, and to promote officer safety and performance. 2. Applicable to All Sworn Members. The following guidelines establish the Baltimore Police Department’s (BPD) Professional Appearance Standards and shall apply to both male and female members, unless otherwise specified. These regulations apply to all sworn personnel working in a uniformed capacity (to include uniformed overtime and uniformed secondary employment) and to all sworn personnel in court attire. GENERAL 1. Unless otherwise authorized, all on-duty sworn personnel shall appear neat, clean and well−groomed and shall project an image of authority, safety and professionalism to the public at all times. 2. All members are required to familiarize themselves with these standards and to remain in strict compliance at all times. Supervisor/Shift Commander 1. Inspect subordinates daily to ensure compliance with the Professional Appearance Standards established in this policy. 2. Take corrective action, when needed. Universal Hairstyle Requirements 1. Hair must be neatly groomed and conform to the shape of the head. 2. Only natural hair colors are permitted. Hair colors that are considered extreme, faddish or artificial, such as purple, pink or green are prohibited. 3. The bulk of the hair will not be excessive or present a ragged or unkempt appearance, and shall not: 3.1. -

Beauty Trends 2015
Beauty Trends 2015 HAIR CARE EDITION (U.S.) The image The image cannot be cannot be displayed. displayed. Your Your computer computer may not have may not have enough enough memory to memory to Intro open the open the With every query typed into a search bar, we are given a glimpse into user considerations or intentions. By compiling top searches, we are able to render a strong representation of the United States’ population and gain insight into this specific population’s behavior. In our Google Beauty Trends report, we are excited to bring forth the power of big data into the hands of the marketers, product developers, stylists, trendsetters and tastemakers. The goal of this report is to share useful data for planning purposes accompanied by curated styles of what we believe can make for impactful trends. We are proud to share this iteration and look forward to hearing back from you. Flynn Matthews | Principal Industry Analyst, Beauty Olivier Zimmer | Trends Data Scientist Yarden Horwitz | Trends Brand Strategist Photo Credit: Blind Barber (Men’s Hair), Meladee Shea Gammelseter (Women’s Hair), Andrea Grabher/Christian Anwander (Colored Hair), Catface Hair (Box & Twist Braids), Maria Valentino/MCV photo (Goddess Braid) Proprietary + Confidential Methodology QUERY To compile a list of accurate trends within the Jan-13 Aug-13 Jan-14 Aug-14 Jan-15 Aug-15 beauty industry, we pulled top volume queries related to the beauty category and looked at their monthly volume from January 2013 to August 2015. We first removed any seasonal effect, and DE-SEASONALIZED QUERY then measured the year-over-year growth, velocity, and acceleration for each search query.