Survival of Salmonella on Lemon and Lime Slices and Subsequent Transfer to Beverages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
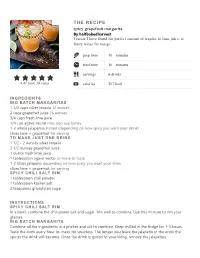
Spicy Grapefruit Margarita. the RECIPE
THE RECIPE spicy grapefruit margarita. By halfbakedharvest I swear I have found the perfect amount of tequila, to lime juice, to fruity mixer for margs prep time 10 minutes total time 10 minutes servings 6 drinks 4.47 from 39 votes calories 207 kcal INGREDIENTS BIG BATCH MARGARITAS 1 1/2 cups silver tequila 12 ounces 2 cups grapefruit juice 16 ounces 3/4 cups fresh lime juice 1/4 cup agave nectar may also use honey 1-2 whole jalapeños halved (depending on how spicy you want your drink) slices lime + grapefruit for serving TO MAKE JUST ONE DRINK 1 1/2 - 2 ounces silver tequila 2 1/2 ounces grapefruit juice 1 ounce fresh lime juice 1 tablespoon agave nectar or more to taste 1-2 slices jalapeno depending on how spicy you want your drink slices lime + grapefruit for serving SPICY CHILI SALT RIM 1 tablespoon chili powder 1 tablespoon kosher salt 2 teaspoons granulated sugar INSTRUCTIONS SPICY CHILI SALT RIM In a bowl, combine the chili power salt and sugar. Mix well to combine. Use this mixture to rim your glasses. BIG BATCH MARGARITA Combine all the ingredients in a pitcher and stir to combine. Keep chilled in the fridge for 1-3 hours. Taste the drink every hour to check for spiciness. The longer you leave the jalapeño in the drink the spicier the drink will become. Once the drink is spiced to your liking, remove the jalapeños. When ready to serve, salt the rims of 4-6 glasses. Add ice to each glass and pour the margaritas over the ice. -

R09 SI: Thermal Properties of Foods
Related Commercial Resources CHAPTER 9 THERMAL PROPERTIES OF FOODS Thermal Properties of Food Constituents ................................. 9.1 Enthalpy .................................................................................... 9.7 Thermal Properties of Foods ..................................................... 9.1 Thermal Conductivity ................................................................ 9.9 Water Content ........................................................................... 9.2 Thermal Diffusivity .................................................................. 9.17 Initial Freezing Point ................................................................. 9.2 Heat of Respiration ................................................................. 9.18 Ice Fraction ............................................................................... 9.2 Transpiration of Fresh Fruits and Vegetables ......................... 9.19 Density ...................................................................................... 9.6 Surface Heat Transfer Coefficient ........................................... 9.25 Specific Heat ............................................................................. 9.6 Symbols ................................................................................... 9.28 HERMAL properties of foods and beverages must be known rizes prediction methods for estimating these thermophysical proper- Tto perform the various heat transfer calculations involved in de- ties and includes examples on the -

Lime (Citrus Aurantifolia (Christm.) Swingle) Essential Oils: Volatile Compounds, Antioxidant Capacity, and Hypolipidemic Effect
foods Article Lime (Citrus aurantifolia (Christm.) Swingle) Essential Oils: Volatile Compounds, Antioxidant Capacity, and Hypolipidemic Effect Li-Yun Lin 1,*, Cheng-Hung Chuang 2 , Hsin-Chun Chen 3 and Kai-Min Yang 4 1 Department of Food Science and Technology, Hungkuang University, Taichung 433, Taiwan 2 Department of Nutrition, Hungkuang University, Taichung 433, Taiwan 3 Department of Cosmeceutics, China Medical University, Taichung 404, Taiwan 4 Department of Hospitality Management, Mingdao Unicersity, ChangHua 523, Taiwan * Correspondence: [email protected]; Tel.: +886-33-432-793 Received: 19 July 2019; Accepted: 2 September 2019; Published: 7 September 2019 Abstract: Lime peels are mainly obtained from the byproducts of the juice manufacturing industry, which we obtained and used to extract essential oil (2.3%) in order to examine the antioxidant and hypolipidaemic effects. We identified 60 volatile compounds of lime essential oil (LEO) with GC/MS, of which the predominant constituents were limonene, γ-terpinene, and β-pinene. Lime essential oil was measured according to the DPPH assay and ABTS assay, with IC50 values of 2.36 mg/mL and 0.26 mg/mL, respectively. This study also explored the protective effects of LEO against lipid-induced hyperlipidemia in a rat model. Two groups of rats received oral LEO in doses of 0.74 g/100 g and 2.23 g/100 g with their diets. Eight weeks later, we found that the administration of LEO improved the serum total cholesterol, triglyceride, low-density lipoprotein cholesterol, alanine aminotransferase, and aspartate transaminase levels in the hyperlipidemic rats (p < 0.05). Simultaneously, the LEO improved the health of the rats in terms of obesity, atherogenic index, and fatty liver. -

Download Article (PDF)
eFood Vol. 1(2); April (2020), pp. 126–139 DOI: https://doi.org/10.2991/efood.k.200406.001; eISSN 2666-3066 https://www.atlantis-press.com/journals/efood Review Emerging Exotic Fruits: New Functional Foods in the European Market Laura Cornara1,*, Jianbo Xiao2, Antonella Smeriglio3, Domenico Trombetta3, Bruno Burlando4,5 1Department of Earth, Environment and Life Sciences (DISTAV), University of Genova, Corso Europa 26, Genova 16132, Italy 2Institute of Chinese Medical Sciences, State Key Laboratory of Quality Research in Chinese Medicine, University of Macau, Taipa, Macau 3 Department of Chemical, Biological, Pharmaceutical and Environmental Sciences, University of Messina, Viale F. Stagno d’Alcontres 31, Messina 98166, Italy 4Department of Pharmacy (DIFAR), University of Genova, Viale Benedetto XV 3, Genova 16132, Italy 5Biophysics Institute, National Research Council (CNR), via De Marini 6, Genova 16149, Italy ARTICLE INFO ABSTRACT Article History The consumption of exotic fruits is rapidly increasing in European countries. Some of these products have attracted much interest due to their alleged properties of preventing malnutrition, over-nutrition, and disease, maintaining a healthy body. Received 23 February 2020 Accepted 31 March 2020 Scientific studies on these fruits are multiplying, including chemical characterizations and biological investigations onin vitro and in vivo experimental models. This review concerns four edible fruits:Hylocereus undatus (dragon fruit), Annona cherimola Keywords (cherimoya), Citrus australasica (finger lime), andAverrhoa carambola (carambola or star fruit). By screening biomedical Superfruits databases, viz. Scopus, WOS, and PubMed, a total of 131 papers have been selected. Data reveals a wide series of biological functional food effects that confirm traditional medicinal uses or suggest new therapeutic applications. -

Citrus Varieties in Egypt: an Impression
International Research Journal of Applied Sciences Short Communication pISSN: 2663-5577, eISSN: 2663-5585 Citrus Varieties in Egypt: An Impression Waleed Fouad Abobatta Department of Citrus, Horticulture Research Institute, Agriculture Research Center, Egypt ARTICLE INFORMATION ABSTRACT Received: Citrus industry is very important for Egyptian economy, citrus fruit is the leading exportable agricultural product of Egypt and is an important source of national income. Citrus cultivation Accepted: area represents about 29% of the total fruit area, there are different citrus varieties cultivated in Egypt. This work aims to provide a short description of main citrus varieties cultivated in Published: Egypt through providing information about fruit size, maturity periods, seediness and productivity average and main cultivated areas for each variety. However Washington Navel Corresponding Author: and Valencia orange are the main varieties followed by Mandarins group varieties, lemon, Waleed Fouad Abobatta, Balady orange, while other varieties like Grapefruit, Sour orange and Kumquat are cultivated Department of Citrus, in small areas. Horticulture Research Institute, Agriculture Research Center, Egypt Key words: Citrus industry, navel orange, valencia orange, mandarins group, citrus varieties INTRODUCTION Citrus is a genus from Rutaceae family, subfamily Aurantoideae1 and there are several species in this genus; but there are major species such as sweet orange (Citrus sinensis (L.) Osbeck), mandarins group, grapefruits (Citrus paradisi ), lime (Citrus aurantifolia) and sour orange (Citrus aurantium L.)2. Citrus is a diploid genus origin in tropical, subtropical, but now it is produced mainly in arid and semiarid regions. Citrus species are among the most widely grown fruit crops in the world and have a huge market all over the world3. -

Citrus from Seed?
Which citrus fruits will come true to type Orogrande, Tomatera, Fina, Nour, Hernandina, Clementard.) from seed? Ellendale Tom McClendon writes in Hardy Citrus Encore for the South East: Fortune Fremont (50% monoembryonic) “Most common citrus such as oranges, Temple grapefruit, lemons and most mandarins Ugli Umatilla are polyembryonic and will come true to Wilking type. Because most citrus have this trait, Highly polyembryonic citrus types : will mostly hybridization can be very difficult to produce nucellar polyembryonic seeds that will grow true to type. achieve…. This unique characteristic Citrus × aurantiifolia Mexican lime (Key lime, West allows amateurs to grow citrus from seed, Indian lime) something you can’t do with, say, Citrus × insitorum (×Citroncirus webberii) Citranges, such as Rusk, Troyer etc. apples.” [12*] Citrus × jambhiri ‘Rough lemon’, ‘Rangpur’ lime, ‘Otaheite’ lime Monoembryonic (don’t come true) Citrus × limettioides Palestine lime (Indian sweet lime) Citrus × microcarpa ‘Calamondin’ Meyer Lemon Citrus × paradisi Grapefruit (Marsh, Star Ruby, Nagami Kumquat Redblush, Chironja, Smooth Flat Seville) Marumi Kumquat Citrus × sinensis Sweet oranges (Blonde, navel and Pummelos blood oranges) Temple Tangor Citrus amblycarpa 'Nasnaran' mandarin Clementine Mandarin Citrus depressa ‘Shekwasha’ mandarin Citrus karna ‘Karna’, ‘Khatta’ Poncirus Trifoliata Citrus kinokuni ‘Kishu mandarin’ Citrus lycopersicaeformis ‘Kokni’ or ‘Monkey mandarin’ Polyembryonic (come true) Citrus macrophylla ‘Alemow’ Most Oranges Citrus reshni ‘Cleopatra’ mandarin Changshou Kumquat Citrus sunki (Citrus reticulata var. austera) Sour mandarin Meiwa Kumquat (mostly polyembryonic) Citrus trifoliata (Poncirus trifoliata) Trifoliate orange Most Satsumas and Tangerines The following mandarin varieties are polyembryonic: Most Lemons Dancy Most Limes Emperor Grapefruits Empress Tangelos Fairchild Kinnow Highly monoembryonic citrus types: Mediterranean (Avana, Tardivo di Ciaculli) Will produce zygotic monoembryonic seeds that will not Naartje come true to type. -

Citrus Fruits 2020 Summary (August 2020) 3 USDA, National Agricultural Statistics Service
United States Department of Citrus Fruits Agriculture National 2020 Summary Agricultural Statistics Service August 2020 ISSN: 1948-9048 Contents Utilized Citrus Production – United States Chart ................................................................................................................... 6 Citrus Value of Production – United States Chart .................................................................................................................. 6 Citrus Narrative ....................................................................................................................................................................... 7 Citrus Acreage, Production, Utilization, and Value – States and United States: 2017-2018, 2018-2019, and 2019-2020 ........................................................................................................................................................................ 8 Citrus Acreage, Production, Utilization, and Value by Crop – United States: 2017-2018, 2018-2019, and 2019-2020 ........................................................................................................................................................................ 9 Orange Acreage, Yield, Utilization, Price, and Value by Type – States and United States: 2017-2018, 2018-2019, and 2019-2020 ................................................................................................................................................... 10 Bearing Acres of Oranges – United States Chart ................................................................................................................. -

Lemons & Limes
Reasons to Eat Lemons & Limes • Fresh lemon and lime pieces, and juice, have vitamin C, which keep your immune system healthy, preventing colds. • Lemons and limes also have fiber in them, which keeps your digestive system healthy. • Limes have iron in them, which keeps your muscles healthy. • Lemons and limes are low in saturated fat and low in sodium. www.sdharvestofthemonth.org Quick Serving Ideas • Place thinly sliced lemons, peel and all, Lemons & underneath and around fish before cooking. Baking or broiling will soften the slices so that they can be eaten along with the fish. Limes • Combine lemon juice with olive or flax oil, freshly crushed garlic and pepper to make a light and refreshing salad dressing. • To decrease salt consumption, serve lemon wedges with meals, as their tartness makes a great salt substitute. • Add an-easy-to-prepare zing to dinner tonight by tossing: seasoned cooked brown rice with garden peas, chicken pieces, scallions, pumpkin seeds, lime or lime juice and zest. • Squeeze some lime or lemon juice onto an avocado quarter and eat as is. How to Store and Juice Lemons & Limes • Lemons and limes can be stored at room temperature for up to one week. • They can be stored for two-three weeks in the refrigerator. • Lemons and limes are often called for in recipes in the form of juice. They will produce more juice when warmer, so always juice them at room temperature, or place them in a bowl of warm water. • Rolling them under the palm of your hand on a flat surface will also help to extract more juice. -
Holdings of the University of California Citrus Variety Collection 41
Holdings of the University of California Citrus Variety Collection Category Other identifiers CRC VI PI numbera Accession name or descriptionb numberc numberd Sourcee Datef 1. Citron and hybrid 0138-A Indian citron (ops) 539413 India 1912 0138-B Indian citron (ops) 539414 India 1912 0294 Ponderosa “lemon” (probable Citron ´ lemon hybrid) 409 539491 Fawcett’s #127, Florida collection 1914 0648 Orange-citron-hybrid 539238 Mr. Flippen, between Fullerton and Placentia CA 1915 0661 Indian sour citron (ops) (Zamburi) 31981 USDA, Chico Garden 1915 1795 Corsican citron 539415 W.T. Swingle, USDA 1924 2456 Citron or citron hybrid 539416 From CPB 1930 (Came in as Djerok which is Dutch word for “citrus” 2847 Yemen citron 105957 Bureau of Plant Introduction 3055 Bengal citron (ops) (citron hybrid?) 539417 Ed Pollock, NSW, Australia 1954 3174 Unnamed citron 230626 H. Chapot, Rabat, Morocco 1955 3190 Dabbe (ops) 539418 H. Chapot, Rabat, Morocco 1959 3241 Citrus megaloxycarpa (ops) (Bor-tenga) (hybrid) 539446 Fruit Research Station, Burnihat Assam, India 1957 3487 Kulu “lemon” (ops) 539207 A.G. Norman, Botanical Garden, Ann Arbor MI 1963 3518 Citron of Commerce (ops) 539419 John Carpenter, USDCS, Indio CA 1966 3519 Citron of Commerce (ops) 539420 John Carpenter, USDCS, Indio CA 1966 3520 Corsican citron (ops) 539421 John Carpenter, USDCS, Indio CA 1966 3521 Corsican citron (ops) 539422 John Carpenter, USDCS, Indio CA 1966 3522 Diamante citron (ops) 539423 John Carpenter, USDCS, Indio CA 1966 3523 Diamante citron (ops) 539424 John Carpenter, USDCS, Indio -

University of Florida Thesis Or Dissertation Formatting Template
A SENSORY EVALUATION OF CITRUS GREENING-AFFECTED JUICE BLENDS By CHINEDU IKPECHUKWU A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2012 1 © 2012 Chinedu Ikpechukwu 2 To my parents: for their unconditional love, support and encouragement 3 ACKNOWLEDGMENTS I would like to give special thanks to Dr. Renee Goodrich-Schneider, my advisor, for giving me the opportunity to pursue my dream as a food scientist and for her guidance and encouragement in completing my master’s degree. I am very grateful for the expertise and support given to me by members of my committee: Dr. Charles Sims, Dr. Wade Yang and Dr. Tim Spann. I would like to personally thank Eric Dreyer, the taste panel manager, as well as his Taste Panel staff that enabled me complete numerous sensory projects properly and on time. I would also like to thank my lab mates, Lemâne Delva, Devin Lewis, and Gayathri Balakrishnan for all their help and suggestions. Most of all, I cannot thank more profusely my family whose support and faith gave me the strength to accomplish my goals. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS .................................................................................................. 4 LIST OF TABLES ............................................................................................................ 7 ABSTRACT .................................................................................................................... -

New and Noteworthy Citrus Varieties Presentation
New and Noteworthy Citrus Varieties Citrus species & Citrus Relatives Hundreds of varieties available. CITRON Citrus medica • The citron is believed to be one of the original kinds of citrus. • Trees are small and shrubby with an open growth habit. The new growth and flowers are flushed with purple and the trees are sensitive to frost. • Ethrog or Etrog citron is a variety of citron commonly used in the Jewish Feast of Tabernacles. The flesh is pale yellow and acidic, but not very juicy. The fruits hold well on the tree. The aromatic fruit is considerably larger than a lemon. • The yellow rind is glossy, thick and bumpy. Citron rind is traditionally candied for use in holiday fruitcake. Ethrog or Etrog citron CITRON Citrus medica • Buddha’s Hand or Fingered citron is a unique citrus grown mainly as a curiosity. The six to twelve inch fruits are apically split into a varying number of segments that are reminiscent of a human hand. • The rind is yellow and highly fragrant at maturity. The interior of the fruit is solid rind with no flesh or seeds. • Fingered citron fruits usually mature in late fall to early winter and hold moderately well on the tree, but not as well as other citron varieties. Buddha’s Hand or Fingered citron NAVEL ORANGES Citrus sinensis • ‘Washington navel orange’ is also known • ‘Lane Late Navel’ was the first of a as the Bahia. It was imported into the number of late maturing Australian United States in 1870. navel orange bud sport selections of Washington navel imported into • These exceptionally delicious, seedless, California. -

S17P11 Evaluation of Lemon Selections for the Deserts of the United States S17P12 Selection and Field Evaluation of Three New Cu
S17P11 Evaluation of Lemon Selections for the Deserts of the United States Wright G.C.1, and Kahn T.2 1University of Arizona (Univ of AZ), Yuma Agriculture Center, USA; and 2University of California Riverside, Department of Botany and Plant Sciences, USA. [email protected] New lemon selections suitable for the US desert climate are needed to diversify production. Desert lemons occupy an early-season market niche and are a source of fruit for packinghouses located in the region and in cooler areas. This project was designed to evaluate 12 lemon selections under desert conditions. The objectives are to provide the lemon industry with information on tree growth, yield, packout, and fruit quality characteristics for selections. These include: ‘Allen Eureka’, ‘Variegated Pink-Fleshed Eureka’, ‘Corona Foothills’ (a bud sport of ‘Villafranca’), ‘Limoneira 8A’ Lisbon’, ‘Walker Lisbon’, ‘Femminello Santa Teresa’, ‘Interdonato’, ‘Limonero Fino 49’, Limonero Fino 95’, ‘Messina’, ‘Seedless’ lemon and ‘Yen Ben’. ‘Corona Foothills’, ‘Limonero Fino 49’, ‘Walker Lisbon’ and ‘Femminello Santa Teresa’ have heretofore had the greatest yields, while ‘Messina’, and ‘Variegated Pink-Fleshed Eureka’ have had the least yield. ‘Yen Ben’ had adequate yields, but had later maturing fruit than most of the other selections. With regards to fruit packout, ‘Messina’ had the largest size, followed by ‘Corona Foothills’, ‘Interdonato’, ‘Limonero Fino 49’ and ‘Limonero Fino 95’. ‘Variegated Pink-Fleshed Eureka’ and ‘Yen Ben’ had the smallest sized fruit. There was little effect of selection upon fruit exterior quality. ‘Variegated Pink Eureka’, ‘Yen Ben’, ‘Messina’ and ‘Seedless’ had the least number of seeds per fruit, while ‘Interdonato’ had the most, followed by ‘Walker Lisbon’, ‘Corona Foothills’, ‘Femminello Santa Teresa’ and ‘Limonero Fino 49’.