Lea Et Al 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Directing an Artificial Zinc Finger Protein to New Targets by Fusion to a Non-DNA Binding Domain
Directing an artificial zinc finger protein to new targets by fusion to a non-DNA binding domain Wooi Fang (Catheryn) Lim A thesis in fulfilment of the requirements for the degree of Doctor of Philosophy School of Biotechnology and Biomolecular Sciences Faculty of Science March 2016 Page | 0 THESIS/ DISSERTATION SHEET Page | i ORIGINALITY STATEMENT ‘I hereby declare that this submission is my own work and to the best of my knowledge it contains no materials previously published or written by another person, or substantial proportions of material which have been accepted for the award of any other degree or diploma at UNSW or any other educational institution, except where due acknowledgement is made in the thesis. Any contribution made to the research by others, with whom I have worked at UNSW or elsewhere, is explicitly acknowledged in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in the project's design and conception or in style, presentation and linguistic expression is acknowledged.’ WOOI FANG LIM Signed …………………………………………….............. 31-03-2016 Date …………………………………………….............. Page | i COPYRIGHT STATEMENT ‘I hereby grant the University of New South Wales or its agents the right to archive and to make available my thesis or dissertation in whole or part in the University libraries in all forms of media, now or here after known, subject to the provisions of the Copyright Act 1968. I retain all proprietary rights, such as patent rights. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. -

Krüppel-Like Factor 17 Upregulates Uterine Corin Expression and Promotes Spiral Artery Remodeling in Pregnancy
Krüppel-like factor 17 upregulates uterine corin expression and promotes spiral artery remodeling in pregnancy Can Wanga, Zhiting Wanga, Meiling Hea, Tiantian Zhoua, Yayan Niua,b, Shengxuan Sunc, Hui Lia, Ce Zhanga, Shengnan Zhanga, Meng Liua, Ying Xud, Ningzheng Donga,b,1, and Qingyu Wua,e,1 aCyrus Tang Hematology Center, Ministry of Education Engineering Center of Hematological Diseases, Collaborative Innovation Center of Hematology, State Key Laboratory of Radiation Medicine and Prevention, Soochow University, 215123 Suzhou, China; bMinistry of Health Key Laboratory of Thrombosis and Hemostasis, Jiangsu Institute of Hematology, The First Affiliated Hospital of Soochow University, 215006 Suzhou, China; cDepartment of Orthopedics, The Second Affiliated Hospital of Soochow University, 215004 Suzhou, China; dCambridge-Soochow University Genomic Resource Center, Soochow University, 215123 Suzhou, China; and eCardiovascular & Metabolic Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195 Edited by R. Michael Roberts, University of Missouri, Columbia, MO, and approved June 25, 2020 (received for review February 29, 2020) Spiral artery remodeling is an important physiological process in the In the heart, natriuretic peptide expression is controlled by pregnant uterus which increases blood flow to the fetus. Impaired GATA-4, a zinc finger transcription factor (21, 22). A similar spiral artery remodeling contributes to preeclampsia, a major disease GATA-4–dependent mechanism is critical for cardiac corin ex- in pregnancy. Corin, a transmembrane serine protease, is up- pression. We have shown that human and mouse corin gene pro- regulated in the pregnant uterus to promote spiral artery remodeling. moters contain a conserved sequence that is essential for GATA-4 To date, the mechanism underlying uterine corin up-regulation re- binding and corin expression in cardiomyocytes (23). -

Engineered Type 1 Regulatory T Cells Designed for Clinical Use Kill Primary
ARTICLE Acute Myeloid Leukemia Engineered type 1 regulatory T cells designed Ferrata Storti Foundation for clinical use kill primary pediatric acute myeloid leukemia cells Brandon Cieniewicz,1* Molly Javier Uyeda,1,2* Ping (Pauline) Chen,1 Ece Canan Sayitoglu,1 Jeffrey Mao-Hwa Liu,1 Grazia Andolfi,3 Katharine Greenthal,1 Alice Bertaina,1,4 Silvia Gregori,3 Rosa Bacchetta,1,4 Norman James Lacayo,1 Alma-Martina Cepika1,4# and Maria Grazia Roncarolo1,2,4# Haematologica 2021 Volume 106(10):2588-2597 1Department of Pediatrics, Division of Stem Cell Transplantation and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 2Stanford Institute for Stem Cell Biology and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 3San Raffaele Telethon Institute for Gene Therapy, Milan, Italy and 4Center for Definitive and Curative Medicine, Stanford School of Medicine, Stanford, CA, USA *BC and MJU contributed equally as co-first authors #AMC and MGR contributed equally as co-senior authors ABSTRACT ype 1 regulatory (Tr1) T cells induced by enforced expression of interleukin-10 (LV-10) are being developed as a novel treatment for Tchemotherapy-resistant myeloid leukemias. In vivo, LV-10 cells do not cause graft-versus-host disease while mediating graft-versus-leukemia effect against adult acute myeloid leukemia (AML). Since pediatric AML (pAML) and adult AML are different on a genetic and epigenetic level, we investigate herein whether LV-10 cells also efficiently kill pAML cells. We show that the majority of primary pAML are killed by LV-10 cells, with different levels of sensitivity to killing. Transcriptionally, pAML sensitive to LV-10 killing expressed a myeloid maturation signature. -

1 Dynamics of Cardiomyocyte Transcriptome and Chromatin
bioRxiv preprint doi: https://doi.org/10.1101/488593; this version posted December 7, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 DYNAMICS OF CARDIOMYOCYTE TRANSCRIPTOME AND CHROMATIN 2 LANDSCAPE DEMARCATES KEY EVENTS OF HEART DEVELOPMENT 3 4 Pawlak Michal1, Kedzierska Katarzyna Z.1, Migdal Maciej1, Abu Nahia Karim1, Ramilowski Jordan 5 A.3, Bugajski Lukasz2, Hashimoto Kosuke3, Marconi Aleksandra1, Piwocka Katarzyna2, Carninci 6 Piero3, Winata Cecilia L.1,4* 7 8 1International Institute of Molecular and Cell Biology in Warsaw, Laboratory of Zebrafish 9 Developmental Genomics, Warsaw, Poland; 2Nencki Institute of Experimental Biology, Laboratory of 10 Cytometry, Warsaw, Poland; 3RIKEN Center for Integrative Medical Sciences, RIKEN Yokohama 11 Institute, Japan; 4Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany 12 13 *International Institute of Molecular Biology in Warsaw 14 4 Ks. Trojdena Street 15 02 - 109 Warsaw, Poland 16 [email protected] 17 Running title: Regulatory landscape of heart development 18 Keywords: heart development, CHD, chromatin, transcriptomics, ATAC-seq, RNA-seq, genomics, 19 epigenomics, zebrafish, NGS. 20 21 22 23 24 25 26 27 1 bioRxiv preprint doi: https://doi.org/10.1101/488593; this version posted December 7, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. -

Labinvest20154.Pdf
12A ANNUAL MEETING ABSTRACTS (0-4 d), exposure to corticosteroids, or days of alcohol fixation (1-53 d). The alcohol by the Ministry of Health, Labour and Welfare ever since the initiation of registration fixed cases with the longest fixation times (41-53 d) showed variable degeneration of of all maternal deaths to the Japan Association of Obstetricians and Gynecologists in the RC. Immunostains for androgens stained Leydig cells, but not the RC. 2010. There were 147 cases of maternal death registered in Japan (2011-2013), with Conclusions: RC are very common, probably ubiquitous in normal testicles, but their the maternal mortality rate being 3.9 per 100,000 live births as the average annual live number is variable. They show amphiphilic properties, dissolving rapidly in aqueous birth count was 1039279. Of the 147 cases, 59 autopsies were performed (Autopsy solutions (10% formalin). RC are not common in cytologic preparations from normal rate: 40.1%); in 51 of the 59 autopsies, assessment by the Committee was completed. testes, and their presence suggests the presence of a LCT. Immunostains for androgens In this study, we analyzed all 51 registered autopsy cases (2011-2013) and classified stain specifically the Leydig cells, but not RC. the causes of maternal death. Design: We analyzed all autopsy reports and medical records in the 51 cases. In 29 Age-Related EBV-Associated Lymphoproliferative Disorder With suspected cases of AFE, we measured the serum levels of zinc-coproporphyrin-1 and Widespread Gastrointestinal Involvement and Subsequent Development sialyl-Tn to detect substances specific to amniotic fluid in maternal blood. -

KLF2 in Regulation of NF-B-Mediated Immune Cell Function And
International Journal of Molecular Sciences Review KLF2 in Regulation of NF-κB-Mediated Immune Cell Function and Inflammation Prerana Jha and Hiranmoy Das * ID Vascular Biology and Stem Cell Research Laboratory, Department of Biomedical Sciences, School of Pharmacy, Texas Tech University Health Sciences Center, Amarillo, TX 79106, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-806-414-9623; Fax: +1-806-356-4770 Received: 23 September 2017; Accepted: 8 November 2017; Published: 10 November 2017 Abstract: KLF2 (Kruppel-like factor 2) is a member of the zinc finger transcription factor family, which critically regulates embryonic lung development, function of endothelial cells and maintenance of quiescence in T-cells and monocytes. It is expressed in naïve T-cells and monocytes, however its level of expression decreases during activation and differentiation. KLF2 also plays critical regulatory role in various inflammatory diseases and their pathogenesis. Nuclear factor-kappaB (NF-κB) is an important inducer of inflammation and the inflammation is mediated through the transcription of several proinflammatory cytokines, chemokines and adhesion molecules. So, both transcriptional factors KLF2 and NF-κB are being associated with the similar cellular functions and their maintenance. It was shown that KLF2 regulates most of the NF-κB-mediated activities. In this review, we focused on emphasizing the involvement of KLF2 in health and disease states and how they interact with transcriptional master regulator NF-κB. Keywords: KLF2; NF-κB; immune cells; inflammation and diseases 1. Introduction Kruppel-like factor (KLF)s are the member of zinc finger family of DNA-binding transcription factors, which play diverse role in various biological processes including quiescence, proliferation, differentiation, development, growth and inflammation [1,2]. -
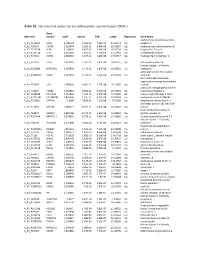
Table S1. the Statistical Metrics for Key Differentially Expressed Genes (Degs)
Table S1. The statistical metrics for key differentially expressed genes (DEGs) Gene Agilent Id Symbol logFC pValue FDR tvalue Regulation Gene Name oxidized low density lipoprotein A_24_P124624 OLR1 2.458429 1.19E-13 7.25E-10 24.04241 Up receptor 1 A_23_P90273 CHST8 2.622464 3.85E-12 6.96E-09 19.05867 Up carbohydrate sulfotransferase 8 A_23_P217528 KLF8 2.109007 4.85E-12 7.64E-09 18.76234 Up Kruppel like factor 8 A_23_P114740 CFH 2.651636 1.85E-11 1.79E-08 17.13652 Up complement factor H A_23_P34031 XAGE2 2.000935 2.04E-11 1.81E-08 17.02457 Up X antigen family member 2 A_23_P27332 TCF4 1.613097 2.32E-11 1.87E-08 16.87275 Up transcription factor 4 histone cluster 1 H1 family A_23_P250385 HIST1H1B 2.298658 2.47E-11 1.87E-08 16.80362 Up member b abnormal spindle microtubule A_33_P3288159 ASPM 2.162032 2.79E-11 2.01E-08 16.66292 Up assembly H19, imprinted maternally expressed transcript (non-protein A_24_P52697 H19 1.499364 4.09E-11 2.76E-08 16.23387 Up coding) potassium voltage-gated channel A_24_P31627 KCNB1 2.289689 6.65E-11 3.97E-08 15.70253 Up subfamily B member 1 A_23_P214168 COL12A1 2.155835 7.59E-11 4.15E-08 15.56005 Up collagen type XII alpha 1 chain A_33_P3271341 LOC388282 2.859496 7.61E-11 4.15E-08 15.55704 Up uncharacterized LOC388282 A_32_P150891 DIAPH3 2.2068 7.83E-11 4.22E-08 15.5268 Up diaphanous related formin 3 zinc finger protein 185 with LIM A_23_P11025 ZNF185 1.385721 8.74E-11 4.59E-08 15.41041 Up domain heat shock protein family B A_23_P96872 HSPB11 1.887166 8.94E-11 4.64E-08 15.38599 Up (small) member 11 A_23_P107454 -

Supplementary Figure S1 A
Supplementary Figure S1 A B C 1 4 1 3 1 2 1 2 1 0 1 1 (Normalized signal values)] 2 (Normalized signal values) (Normalized 8 2 10 Log 6 AFFX-BioC AFFX-BioB AFFX-BioDn AFFX-CreX hDFs [Log 6 8 10 12 14 hOFs [Log2 (Normalized signal values)] Supplementary Figure S2 GLYCOLYSIS PENTOSE-PHOSPHATE PATHWAY Glucose Purine/pyrimidine Glucose-6-phosphate metabolism AMINO ACID Fluctose-6-phosphate AMPK METABOLISM TIGAR PFKFB2 methylgloxal GloI Ser, Gly, Thr Glyceraldehyde-3-phosphate ALDH Lactate PYRUVATE LDH METABOLISM acetic acid Ethanol Pyruvate GLYCOSPHINGOLIPID NADH BIOSYNTHESIS Ala, Cys DLD PDH PDK3 DLAT Fatty acid Lys, Trp, Leu, Acetyl CoA ACAT2 Ile, Tyr, Phe β-OXIDATION ACACA Citrate Asp, Asn Citrate Acetyl CoA Oxaloacetate Isocitrate MDH1 IDH1 Glu, Gln, His, ME2 TCA Pro, Arg 2-Oxoglutarate MDH1 CYCLE Pyruvate Malate ME2 GLUTAMINOLYSIS FH Succinyl-CoA Fumalate SUCLA2 Tyr, Phe Var, Ile, Met Supplementary Figure S3 Entrez Gene Symbol Gene Name hODs hDFs hOF-iPSCs GeneID 644 BLVRA biliverdin reductase A 223.9 259.3 253.0 3162 HMOX1 heme oxygenase 1 1474.2 2698.0 452.3 9365 KL klotho 54.1 44.8 36.5 nicotinamide 10135 NAMPT 827.7 626.2 2999.8 phosphoribosyltransferase nuclear factor (erythroid- 4780 NFE2L2 2134.5 1331.7 1006.2 derived 2) related factor 2 peroxisome proliferator- 5467 PPARD 1534.6 1352.9 330.8 activated receptor delta peroxisome proliferator- 5468 PPARG 524.4 100.8 63.0 activated receptor gamma 5621 PRNP prion protein 4059.0 3134.1 1065.5 5925 RB1 retinoblastoma 1 882.9 805.8 739.3 23411 SIRT1 sirtuin 1 231.5 216.8 1676.0 7157 TP53 -

Systemic Mesenchymal Stem Cell Treatment Mitigates Structural and Functional Retinal Ganglion Cell Degeneration in a Mouse Model of Multiple Sclerosis
Article Systemic Mesenchymal Stem Cell Treatment Mitigates Structural and Functional Retinal Ganglion Cell Degeneration in a Mouse Model of Multiple Sclerosis Oliver W. Gramlich1,2,AlexanderJ.Brown3,4, Cheyanne R. Godwin1,2, Michael S. Chimenti5, Lauren K. Boland6, James A. Ankrum6, and Randy H. Kardon1,2 1 Department of Ophthalmology and Visual Sciences, The University of Iowa, Iowa City, IA, USA 2 Center for the Prevention and Treatment of Visual Loss, Iowa City VA Health Care System, Iowa City, IA, USA 3 Department of Biomedical Research, National Jewish Health, Denver, CO, USA 4 Department of Immunology & Microbiology, University of Colorado Anschutz Medical Campus, Aurora, CO, USA 5 Iowa Institute of Human Genetics, Carver College of Medicine, The University of Iowa, Iowa City, IA, USA 6 Roy J. Carver Department of Biomedical Engineering College, The University of Iowa, Iowa City, IA, USA Correspondence: Oliver W. Purpose: The purpose of this study was to determine mesenchymal stem cell (MSC) Gramlich, Department of therapy efficacy on rescuing the visual system in the experimental autoimmune Ophthalmology and Visual Sciences, encephalomyelitis (EAE) model of multiple sclerosis (MS) and to provide new mecha- The University of Iowa, 200 Hawkins nistic insights. Dr, Iowa City, IA 52242, USA. e-mail: Methods: [email protected] EAE was induced in female C57BL6 mice by immunization with myelin oligo- dendrocyte glycoprotein (MOG)35–55, complete Freund’s adjuvant, and pertussis toxin. Received: March 23, 2020 The findings were compared to sham-immunized mice. Half of the EAE mice received Accepted: May 26, 2020 intraperitoneally delivered stem cells (EAE + MSC). -

Transcriptome Analysis of Ageing in Uninjured Human Achilles Tendon
Peffers et al. Arthritis Research & Therapy (2015) 17:33 DOI 10.1186/s13075-015-0544-2 RESEARCH ARTICLE Open Access Transcriptome analysis of ageing in uninjured human Achilles tendon Mandy Jayne Peffers1*, Yongxiang Fang2, Kathleen Cheung3, Tim Koh Jia Wei4, Peter David Clegg1 and Helen Lucy Birch5 Abstract Introduction: The risk of tendon injury and disease increases significantly with increasing age. The aim of the study was to characterise transcriptional changes in human Achilles tendon during the ageing process in order to identify molecular signatures that might contribute to age-related degeneration. Methods: RNA for gene expression analysis using RNA-Seq and quantitative real-time polymerase chain reaction analysis was isolated from young and old macroscopically normal human Achilles tendon. RNA sequence libraries were prepared following ribosomal RNA depletion, and sequencing was undertaken by using the Illumina HiSeq 2000 platform. Expression levels among genes were compared by using fragments per kilobase of exon per million fragments mapped. Differentially expressed genes were defined by using Benjamini-Hochberg false discovery rate approach (P <0.05, expression ratios 1.4 log2 fold change). Alternative splicing of exon variants were also examined by using Cufflinks. The functional significance of genes that showed differential expression between young and old tendon was determined by using ingenuity pathway analysis. Results: In total, the expression of 325 transcribed elements, including protein-coding transcripts and non-coding transcripts (small non-coding RNAs, pseudogenes, long non-coding RNAs and a single microRNA), was significantly different in old compared with young tendon (±1.4 log2 fold change, P <0.05). Of these, 191 were at higher levels in older tendon and 134 were at lower levels in older tendon. -

The Role of the Ubiquitously Expressed Transcription Factor Sp1 in Tissue-Specific Transcriptional Regulation and in Disease
YALE JOURNAL OF BIOLOGY AND MEDICINE 89 (2016), pp.513-525. RevIeW The Role of the Ubiquitously Expressed Transcription Factor Sp1 in Tissue-specific Transcriptional Regulation and in Disease Leigh O’Connor *, Jane Gilmour, Constanze Bonifer * Institute of Cancer and Genomic Sciences, Institute of Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, UK Sp1 belongs to the 26 member strong Sp/KLF family of transcription factors. It is a paradigm for a ubiqui - tously expressed transcription factor and is involved in regulating the expression of genes associated with a wide range of cellular processes in mammalian cells. Sp1 can interact with a range of proteins, including other transcription factors, members of the transcription initiation complex and epigenetic regulators, en - abling tight regulation of its target genes. In this review, we discuss the mechanisms involved in Sp1-medi - ated transcriptional regulation, as well as how a ubiquitous transcription factor can be involved in establishing a tissue-specific pattern of gene expression and mechanisms by which its activity may be regu - lated. We also consider the role of Sp1 in human diseases, such as cancer. INTRODUCTION gene for transcription or, in the case of repressors, block - Gene expression needs to be tightly regulated as the ing it. specific pattern of gene activation or repression is deci - Transcription factors interact in a combinatorial sive for establishing fates. The gene expression program fashion to uniquely regulate genes and, in response to dif - of a cell is controlled by the activities and the interac - ferent stimuli, regulate tissue-specific and developmen - tions of the epigenetic regulatory machinery and se - tal stage-specific gene expression.