Oral Vs. Nasal Breathing During Submaximal Aerobic Exercise
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Review Nursingingeneralpractice
The health benefits of nose breathing Item Type Article Authors Allen, Ruth Publisher Nursing in General Practice Journal Nursing in General Practice Download date 01/10/2021 07:15:20 Link to Item http://hdl.handle.net/10147/559021 Find this and similar works at - http://www.lenus.ie/hse clinical review nursingingeneralpractice The health benefits of nose breathing DR Alan RUth, BehaviouRal Medicine PRactitioneR “For breath is life, and if you breathe well you will live long on earth.” sanskrit Proverb For the most part people are unaware of their breathing and take it for granted that they do it correctly. t has been estimated that approximately one third of people ing. However, it has been estimated that up to 30-50% of modern don’t breathe well enough to sustain normal health. These adults breathe through the mouth, especially during the early people do not get enough oxygenation of their cells, tissues morning hours. and organs. In the book Behavioural and Psychological Ap- Mouth breathing is common in individuals whose nasal proaches to Breathing Disorders, Dr Chandra Patel describes passages are blocked or restricted. A deviated nasal septum Ithe problem with breathing as follows: or small nostril size can lead a person to breathe through their “We start life with a breath, and the process continues mouth instead of their nose. However, breathing through the automatically for the rest of our lives. Because breathing mouth most of the time was not nature’s intention. Many studies continues on its own, without our awareness, it does not have demonstrated that chronic mouth breathing can result in a necessarily mean that it is always functioning for optimum number of adverse health consequences (see Table 1). -

Empty Nose Syndrome
EMPTY NOSE SYNDROME 1 Empty Nose Syndrome 1.1 Definition The descriptive term “Empty nose syndrome” (ENS) was first coined by Kern and Stenkvist in 1994, who described the condition with substantial loss of tissue inside the nose in the region of the inferior and middle turbinates as “empty nose” (Scheithauer, 2010). “Empty nose” patients suffer from chronic nasal crusting, dryness, and intermittent bleeding. The most distinctive symptom of ENS is “paradoxical obstruction”- in that the patient suffers from difficulty breathing despite a widely patent nasal airway (Sozansky & Houser, 2014). ENS patients describe their feeling of nasal obstruction as constant and continuous: feeling of suffocation, inability or significant difficulty to breathe through their nose, feeling that their nose is too open, sensation of excessive airflow, shortness of breath, difficulty to properly inflate the lungs, lack of nasal resistance, and/or undifferentiated breathing difficulties (Houser, 2006; Houser, 2007; Chhabra & Houser, 2009; Scheithauer, 2010; Coste et al, 2012). 1.2 Primary symptoms ENS patients may experience various debilitating and devastating symptoms, in varying degrees, that can occur after excessive resection of functioning turbinate tissue from the nasal cavity (Moore & Kern, 2001). While the primary symptom is nasal airway obstruction, other symptoms depend upon the type of surgery, extent of functional nasal tissue removal, nature of the bacterial organisms growing inside the nasal cavity, and other unidentified factors. The set and level of severity of symptoms depends upon each individual case. ENS-related symptoms can be divided into three major categories and may include: Respiratory: paradoxical obstruction (feeling of suffocation, inability or significant difficulty to breathe through the nose, feeling that the nose is too open, sensation of excessive airflow, lack of nasal resistance, shortness of breath (dyspnea), difficulty to properly inflate the lungs, difficulty drawing a full breath, weakened airflow, and undifferentiated breathing difficulties) 1. -
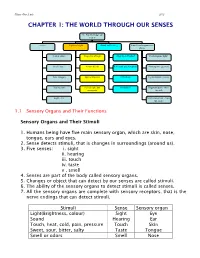
Chapter 1: the World Through Our Senses
Science Form 2 note 2012 CHAPTER 1: THE WORLD THROUGH OUR SENSES The World through our senses senses Light and sight Sound and hearing Stimuli and responses in plants Touch (skin) Properties of light Properties of sound Phototropism (light) Smell (nose) Vision defects Reflection and absorption Geotropism (gravity) Taste (tongue) Optical illusions limitations Hydrotropism (water) Hearing (ear) Stereoscopic and stereophonic Thigmotropism (move monocular toward) Sight (eye) Nastic movement (move run away) 1.1 Sensory Organs and Their Functions Sensory Organs and Their Stimuli 1. Humans being have five main sensory organ, which are skin, nose, tongue, ears and eyes. 2. Sense detects stimuli, that is changes in surroundings (around us). 3. Five senses: i. sight ii. hearing iii. touch iv. taste v . smell 4. Senses are part of the body called sensory organs. 5. Changes or object that can detect by our senses are called stimuli. 6. The ability of the sensory organs to detect stimuli is called senses. 7. All the sensory organs are complete with sensory receptors, that is the nerve endings that can detect stimuli. Stimuli Sense Sensory organ Light(Brightness, colour) Sight Eye Sound Hearing Ear Touch, heat, cold, pain, pressure Touch Skin Sweet, sour, bitter, salty Taste Tongue Smell or odors Smell Nose Science Form 2 note 2012 Laman web. http://freda.auyeung.net/5senses/see.htm http://freda.auyeung.net/5senses/touch.htm http://freda.auyeung.net/5senses/hear.htm http://freda.auyeung.net/5senses/taste.htm http://freda.auyeung.net/5senses/smell.htm 1.2 The Pathway from Stimulus to Response PMR 05 Stimulus Sensory organs Nerves Brain Nerve Response Figure 1.2 The summary of the pathway from stimulus to response 1. -

Toxicological Profile for Acetone Draft for Public Comment
ACETONE 1 Toxicological Profile for Acetone Draft for Public Comment July 2021 ***DRAFT FOR PUBLIC COMMENT*** ACETONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. This information is distributed solely for the purpose of pre dissemination public comment under applicable information quality guidelines. It has not been formally disseminated by the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy. ***DRAFT FOR PUBLIC COMMENT*** ACETONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. -
Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses NOSE Version 1.0 2018-08-30
NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the NNOSEasal Cavity and Paranasal Sinuses NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses NOSE Version 1.0 2018-08-30 NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation Version 1.0 of Airflow in the Nasal Cavity and Paranasal Sinuses NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses Project Number 1000043433 Project Title NOSE Pilot Study Document Reference Investigating the Practical Use of Computational Date 2018-08-30 Title Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses Document Name NOSE_PS_FINAL_v1 Version Draft draft final Restrictions public internal restricted : Distribution Steirische Forschungsförderung Authors Koch Walter, Koch Gerda, Vitiello Massimo, Ortiz Ramiro, Stockklauser Jutta, Benda Odo Abstract The NOSE Pilot study evaluated the technical and scientific environment required for establishing a service portfolio that includes CFD simulation and 3D visualization services for ENT specialists. For this purpose the state-of-the-art of these technologies and their use for upper airways diagnostics were analysed. Keywords Rhinology, Computational Fluid Dynamics, 3D Visualization, Clinical Pathways, Service Center, Knowledge Base Document Revisions Version Date Author(s) Description of Change 1.0 2018-08-30 Koch Walter, Koch Final Version Gerda, Vitiello Massimo, Ortiz NOSERamiro, Stockklauser Jutta, Benda Odo 2018-08-30 Seite 3 / 82 Copyright © AIT ForschungsgesmbH NOSE_PS_FINAL_v1 NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation Version 1.0 of Airflow in the Nasal Cavity and Paranasal Sinuses Table of Contents Acknowledgments .................................................................................. -

Physical and Geometric Constraints Shape the Labyrinth-Like Nasal Cavity
Physical and geometric constraints shape the labyrinth-like nasal cavity David Zwickera,b,1, Rodolfo Ostilla-Monico´ a,b, Daniel E. Liebermanc, and Michael P. Brennera,b aJohn A. Paulson School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138; bKavli Institute for Bionano Science and Technology, Harvard University, Cambridge, MA 02138; and cDepartment of Human Evolutionary Biology, Harvard University, Cambridge, MA 02138 Edited by Leslie Greengard, New York University, New York, NY, and approved January 26, 2018 (received for review August 29, 2017) The nasal cavity is a vital component of the respiratory system take into account geometric constraints imposed by the shape that heats and humidifies inhaled air in all vertebrates. Despite of the head that determine the length of the nasal cavity, its this common function, the shapes of nasal cavities vary widely cross-sectional area, and, generally, the shape of the space that it across animals. To understand this variability, we here connect occupies. To tackle this complex problem, we first show that, nasal geometry to its function by theoretically studying the air- without geometric constraints, optimal shapes have slender flow and the associated scalar exchange that describes heating cross-sections. We then demonstrate that these shapes can be and humidification. We find that optimal geometries, which have compacted into the typical labyrinth-like shapes without much minimal resistance for a given exchange efficiency, have a con- loss in performance. stant gap width between their side walls, while their overall shape can adhere to the geometric constraints imposed by the Results head. Our theory explains the geometric variations of natural The Flow in the Nasal Cavity Is Laminar. -

Facial Genetics: a Brief Overview
fgene-09-00462 October 16, 2018 Time: 12:3 # 1 REVIEW published: 16 October 2018 doi: 10.3389/fgene.2018.00462 Facial Genetics: A Brief Overview Stephen Richmond1*, Laurence J. Howe2,3, Sarah Lewis2,4, Evie Stergiakouli2,4 and Alexei Zhurov1 1 Applied Clinical Research and Public Health, School of Dentistry, College of Biomedical and Life Sciences, Cardiff University, Cardiff, United Kingdom, 2 MRC Integrative Epidemiology Unit, Population Health Sciences, University of Bristol, Bristol, United Kingdom, 3 Institute of Cardiovascular Science, University College London, London, United Kingdom, 4 School of Oral and Dental Sciences, University of Bristol, Bristol, United Kingdom Historically, craniofacial genetic research has understandably focused on identifying the causes of craniofacial anomalies and it has only been within the last 10 years, that there has been a drive to detail the biological basis of normal-range facial variation. This initiative has been facilitated by the availability of low-cost hi-resolution three- dimensional systems which have the ability to capture the facial details of thousands of individuals quickly and accurately. Simultaneous advances in genotyping technology have enabled the exploration of genetic influences on facial phenotypes, both in the Edited by: present day and across human history. Peter Claes, KU Leuven, Belgium There are several important reasons for exploring the genetics of normal-range variation Reviewed by: in facial morphology. Hui-Qi Qu, Children’s Hospital of Philadelphia, - Disentangling -

Computational Analysis of Airflow and Particle Deposition Fraction in The
Biology, Engineering and Medicine Research Article ISSN: 2399-9632 Computational analysis of airflow and particle deposition fraction in the upper part of the human respiratory system Saghaian SE1*, Azimian AR2, Jalilvand R3, Dadkhah S4 and Saghaian SM5 1Department of Mechanical Engineering, University of Kentucky, Lexington, KY 40506, USA 2Department of Mechanical Engineering, Islamic Azad University Khomeini Shahr Branch, Isfahan, Iran 3Department of Mechanical Engineering, Isfahan University of Technology, Isfahan, Iran 4Department of Biology, University of Kentucky, Lexington, KY 40508, USA 5Department of Mechanical, Materials and Aerospace Engineering, Illinois Institute of Technology, Chicago, IL 60616, USA Abstract This research investigates the computational analysis of airflow during inspiration and the tracking of particles in the human respiratory system from the nasal cavity to the third generation of the lungs. Also, the effect of geometric features of nasal cavities on their airflow is evaluated in this study. The actual geometry of the respiratory tract was extracted from CT-Scan medical images of a healthy person using image processing software. The flow is evaluated in laminar and steady states. The airflow rate at nasal entrances was assumed to be 20 L/min, which is related to sitting and resting situations. The movement and deposition of particles with a diameter of 1-30 micrometers in different parts of the human respiratory system have also been evaluated. The fluid field and movements of solid particles were evaluated by Eulerian and Lagrangian methods, respectively. Based on the obtained results, the greater the diameter of the particles, the more particles will be deposited in the nasal cavity. Introduction The research of Zachow et al. -

Nasal Breathing No Tongue Habits Lip Seal Corruption of the “Big 3” Is the Root Cause of Nearly All Malocclusions
Timothy Gross, DMD Part One In the Physiologic Orthodontics class at the Las Vegas Institute, we focus heavily on the “Big 3.” Orthodontic treatment and post treatment orthodontic stability necessitate management and correction of the “Big 3.” So, what is the “Big 3?” 1 2 3 Nasal Breathing No Tongue Habits Lip Seal Corruption of the “Big 3” is the root cause of nearly all malocclusions. That’s right. Class I, Class II, and Class III malocclusions, crowding, deficient midfaces, cross bites, impacted teeth, high palatal arches, deep bites, open bites (and the list goes on) can all be explained by Enlow in his textbook, “The Essentials of Facial Growth.” That textbook is paramount for understanding normal facial growth and development and should be required reading for every dentist and dental specialist. Malocclusions, so predominant in our society, cannot be explained by genetics alone. Our phenotype, i.e. our genome in addition to environmental influences, is the result of corruption of the “Big 3.” The inability to breathe nasally, habitual mouth straight and make the upper and lower teeth couple breathing, and/or tongue habits lead to altered nicely. Unfortunately, the result is a skeletal and facial growth patterns which in turn lead to physiologic malocclusion with straight teeth, at observable dental and orthognathic malocclusions. the expense of the airway, the temporomandibular Orthodontic treatment is utilized to correct these joints and facial aesthetics. malocclusions, but the underlying pathophysiology must be addressed in order to achieve an optimal A Case Study… Me physiologic occlusion, balanced facial growth and As a 48 year old male, I had multiple issues: a facial beauty. -

Lecture 14 --Olfaction.Pdf
14 Olfaction ClickChapter to edit 14 MasterOlfaction title style • Olfactory Physiology • Neurophysiology of Olfaction • From Chemicals to Smells • Olfactory Psychophysics, Identification, and Adaptation • Olfactory Hedonics • Associative Learning and Emotion: Neuroanatomical and Evolutionary Considerations ClickIntroduction to edit Master title style Olfaction: The sense of smell Gustation: The sense of taste ClickOlfactory to edit Physiology Master title style Odor: The translation of a chemical stimulus into a smell sensation. Odorant: A molecule that is defined by its physiochemical characteristics, which are capable of being translated by the nervous system into the perception of smell. To be smelled, odorants must be: • Volatile (able to float through the air) • Small • Hydrophobic (repellent to water) Figure 14.1 Odorants ClickOlfactory to edit Physiology Master title style The human olfactory apparatus • Unlike other senses, smell is tacked onto an organ with another purpose— the nose. Primary purpose—to filter, warm, and humidify air we breathe . Nose contains small ridges, olfactory cleft, and olfactory epithelium ClickOlfactory to edit Physiology Master title style The human olfactory apparatus (continued) • Olfactory cleft: A narrow space at the back of the nose into which air flows, where the main olfactory epithelium is located. • Olfactory epithelium: A secretory mucous membrane in the human nose whose primary function is to detect odorants in inhaled air. Figure 14.2 The nose ClickOlfactory to edit Physiology Master title style Olfactory epithelium: The “retina” of the nose • Three types of cells . Supporting cells: Provide metabolic and physical support for the olfactory sensory neurons. Basal cells: Precursor cells to olfactory sensory neurons. Olfactory sensory neurons (OSNs): The main cell type in the olfactory epithelium. -

In Vitro Evaluation of Nasal Aerosol Depositions: an Insight for Direct Nose to Brain Drug Delivery
pharmaceutics Review In Vitro Evaluation of Nasal Aerosol Depositions: An Insight for Direct Nose to Brain Drug Delivery Aida Maaz, Ian S. Blagbrough * and Paul A. De Bank Department of Pharmacy and Pharmacology, University of Bath, Bath BA2 7AY, UK; [email protected] (A.M.); [email protected] (P.A.D.B.) * Correspondence: [email protected]; Tel.: +44-1225-386-795 Abstract: The nasal cavity is an attractive route for both local and systemic drug delivery and holds great potential for access to the brain via the olfactory region, an area where the blood–brain barrier (BBB) is effectively absent. However, the olfactory region is located at the roof of the nasal cavity and only represents ~5–7% of the epithelial surface area, presenting significant challenges for the deposition of drug molecules for nose to brain drug delivery (NTBDD). Aerosolized particles have the potential to be directed to the olfactory region, but their specific deposition within this area is confounded by a complex combination of factors, which include the properties of the formulation, the delivery device and how it is used, and differences in inter-patient physiology. In this review, an in-depth examination of these different factors is provided in relation to both in vitro and in vivo studies and how advances in the fabrication of nasal cast models and analysis of aerosol deposition can be utilized to predict in vivo outcomes more accurately. The challenges faced in assessing the nasal deposition of aerosolized particles within the paediatric population are specifically considered, representing an unmet need for nasal and NTBDD to treat CNS disorders. -

Particle Deposition and Resistance in the Noses of Adults and Children
Eur Resplr J 1991, 4, 694-702 Particle deposition and resistance in the noses of adults and children M.H. Becquemin*, D.L. Swift**, A Bouchikhi*, M. Roy***, A. Teillac* Particle deposition and resistance in the noses of adults and children. M.H. • Service Central d'EFR: H~pital Piti~-Salp~triere, Becquemin, D.L. Swift, A. Bouchikhi, M. Roy, A. Teillac. Universit~ Lariboisiere Saint-Louis, Paris, France. ABSTRACT: Nasal filter efficiency for particles has been described by several authors as showing large individual variations, probably somehow •• Johns Hopkins University School of Hygiene and Public Health, Baltimore, MD 21205, USA. related to airflow resistance. Twelve children, aged 5.5-11.5 yrs and 8 aged 12-15 yrs were compared ••• IPSN/DPS • CEN/FAR, B.P. no. 6- 62265 to a group of ten adults. Deposition of polystyrene beads (1, 2.05, 2.8 llm Fontenay-aux-Roses Cedex, France. mass median aerodynamic diameter (MMAD)) was measured by compar ing Inhaled aerosols and exhaled air concentrations, for both nose and Correspondence: Dr M. Roy, CEAIIPSN/DPS/SEAPS, mouth breathing. Ventilation was controlled to scale breathing patterns B.P. no. 6, F-92265 Fontenay-aux-Roses, Cedex, appropriate for each age either at rest or during moderate exercise to France. allow comparison between subjects Jn similar physiological conditions. Anterior nasal resistance (as a function of flow rate) and standard lung Keywords: Adults and children; dosimet.ry models; function were measured for each subject. nasal airways; particle deposition; rhinomanometry. 1 For the same inhalation flow rate of 0.300 l·s· , children bad much Received: March 1990; accepted after revision March 1 higher nasal resistances than the adults, 0.425:~:0.208 kPa·/' ·s under 12 yrs, 7, 1991.