Do Exotic Beavers Engineer Differently in Sub-Antarctic Ecosystems?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter14.Pdf
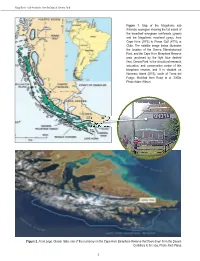
PART I • Omora Park Long-Term Ornithological Research Program THE OMORA PARK LONG-TERM ORNITHOLOGICAL RESEARCH PROGRAM: 1 STUDY SITES AND METHODS RICARDO ROZZI, JAIME E. JIMÉNEZ, FRANCISCA MASSARDO, JUAN CARLOS TORRES-MURA, AND RAJAN RIJAL In January 2000, we initiated a Long-term Ornithological Research Program at Omora Ethnobotanical Park in the world's southernmost forests: the sub-Antarctic forests of the Cape Horn Biosphere Reserve. In this chapter, we first present some key climatic, geographical, and ecological attributes of the Magellanic sub-Antarctic ecoregion compared to subpolar regions of the Northern Hemisphere. We then describe the study sites at Omora Park and other locations on Navarino Island and in the Cape Horn Biosphere Reserve. Finally, we describe the methods, including censuses, and present data for each of the bird species caught in mist nets during the first eleven years (January 2000 to December 2010) of the Omora Park Long-Term Ornithological Research Program. THE MAGELLANIC SUB-ANTARCTIC ECOREGION The contrast between the southwestern end of South America and the subpolar zone of the Northern Hemisphere allows us to more clearly distinguish and appreciate the peculiarities of an ecoregion that until recently remained invisible to the world of science and also for the political administration of Chile. So much so, that this austral region lacked a proper name, and it was generally subsumed under the generic name of Patagonia. For this reason, to distinguish it from Patagonia and from sub-Arctic regions, in the early 2000s we coined the name “Magellanic sub-Antarctic ecoregion” (Rozzi 2002). The Magellanic sub-Antarctic ecoregion extends along the southwestern margin of South America between the Gulf of Penas (47ºS) and Horn Island (56ºS) (Figure 1). -

Regeneration of Subalpine Nothofagus Pumilio in Northwestern Patagonia Argentina
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 2000 Regeneration of subalpine Nothofagus pumilio in northwestern Patagonia Argentina Christopher A. Wall The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Wall, Christopher A., "Regeneration of subalpine Nothofagus pumilio in northwestern Patagonia Argentina" (2000). Graduate Student Theses, Dissertations, & Professional Papers. 6805. https://scholarworks.umt.edu/etd/6805 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. Maureen and Mike MANSFIELD LIBRARY The University of JV IO N T A N A Permission is granted by the author to reproduce this material in its entirety, provided that this material is used for scholarly purposes and is properly cited in published works and reports. ** Please check "Yes" or and provide signature ** Yes, I grant permission k No, I do not grant permission Author’s Signature Date S - P ^ 6 O O Any copying for commercial purposes or financial gain may be undertaken only with the author’s explicit consent. Regeneration of SubalpineNothofagus pumilio in Northwestern Patagonia, Argentina By Christopher A. Wall B.A. Vassar College, 1991 Presented in partial fulfillment of the requirements for the Degree of Master of Science The University of Montana 2000 Approved By Committee Co-Chairs Dean of the Graduate School S'^Zo ' Date UMI Number: EP37606 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. -

Impact of Extreme Weather Events on Aboveground Net Primary Productivity and Sheep Production in the Magellan Region, Southernmost Chilean Patagonia
geosciences Article Impact of Extreme Weather Events on Aboveground Net Primary Productivity and Sheep Production in the Magellan Region, Southernmost Chilean Patagonia Pamela Soto-Rogel 1,* , Juan-Carlos Aravena 2, Wolfgang Jens-Henrik Meier 1, Pamela Gross 3, Claudio Pérez 4, Álvaro González-Reyes 5 and Jussi Griessinger 1 1 Institute of Geography, Friedrich–Alexander-University of Erlangen–Nürnberg, 91054 Erlangen, Germany; [email protected] (W.J.-H.M.); [email protected] (J.G.) 2 Centro de Investigación Gaia Antártica, Universidad de Magallanes, Punta Arenas 6200000, Chile; [email protected] 3 Servicio Agrícola y Ganadero (SAG), Punta Arenas 6200000, Chile; [email protected] 4 Private Consultant, Punta Arenas 6200000, Chile; [email protected] 5 Hémera Centro de Observación de la Tierra, Escuela de Ingeniería Forestal, Facultad de Ciencias, Universidad Mayor, Camino La Pirámide 5750, Huechuraba, Santiago 8580745, Chile; [email protected] * Correspondence: [email protected] Received: 28 June 2020; Accepted: 13 August 2020; Published: 16 August 2020 Abstract: Spatio-temporal patterns of climatic variability have effects on the environmental conditions of a given land territory and consequently determine the evolution of its productive activities. One of the most direct ways to evaluate this relationship is to measure the condition of the vegetation cover and land-use information. In southernmost South America there is a limited number of long-term studies on these matters, an incomplete network of weather stations and almost no database on ecosystems productivity. In the present work, we characterized the climate variability of the Magellan Region, southernmost Chilean Patagonia, for the last 34 years, studying key variables associated with one of its main economic sectors, sheep production, and evaluating the effect of extreme weather events on ecosystem productivity and sheep production. -

Una Nueva Colección De Gavilea Kingii (Hook. F.) MN Corr
Chloris Chilensis 6 (1). 2003. Contenidos Editorial Domínguez, E.: Una nueva colección de Gavilea kingii (Hook. f.) M.N Correa (Orchidaceae) en la Región de Magallanes (XII) Chile. León-Lobos, P.; M.Way, H. Pritchard, A. Moreira-Muñoz, M. León & F. Casado: Conservación ex situ de la flora de Chile en banco de semillas. Novoa, P. & M. Cisternas: Hallazgo de la variedad blanca de Alstroemeria pelegrina L. (Alstroemeriaceae). Pardo, O.: Estudio comparativo de ocho especies americanas de uso medicinal en Mozambique. Pinto, R.: Maihueniopsis nigrispina (Cactaceae-Opuntioidea): nuevo registro para la flora de Chile. Prina, O.A.; G.A. Alfonso & W.A. Muiño: Diversidad de la flora vascular del distrito fitogeográfico de La Payenia, Argentina. Ravenna, P.: Nothofagus macrocarpa y Nothofagus rutila (Fagaceae), dos especies diferentes. Notas F. Schiappacase & P. Peñailillo: Propagación de plantas bulbosas chilenas ornamentales. ¿Cómo enviarnos su artículo? 1 Chloris Chilensis 6 (1). 2003. Miguel Dillon Luis Faúndez Rodolfo Gajardo Comité Editor: Jorge Macaya Carlos Ramírez Sebastián Teillier Año 6. Nº 1. Fecha de Publicación: Junio 2003. ISSN 0717-4632 (Se autoriza la reproducción parcial o total de los artículos, citando la fuente). 2 Chloris Chilensis 6 (1). 2003. CONVOCATORIA Convocar es el verbo exacto para definir el objetivo de esta publicación. En efecto, la idea central de este proyecto de cyber-revista es convocar a los botánicos para participar en estas páginas electrónicas cuyo fin es difundir el conocimiento de la flora y la vegetación de Chile y de los países vecinos. Convocamos a participar en Chloris Chilensis -Revista Chilena de Flora y Vegetación- a todos los botánicos: a los botánicos-biólogos, a los botánicos-profesores, a los botánicos- agrónomos, a los botánicos-forestales, a los botánicos-paisajistas; en fin, a todos quienes tengan algo que publicar de interés para el resto de sus colegas. -

Chile: a Journey to the End of the World in Search of Temperate Rainforest Giants
Eliot Barden Kew Diploma Course 53 July 2017 Chile: A Journey to the end of the world in search of Temperate Rainforest Giants Valdivian Rainforest at Alerce Andino Author May 2017 1 Eliot Barden Kew Diploma Course 53 July 2017 Table of Contents 1. Title Page 2. Contents 3. Table of Figures/Introduction 4. Introduction Continued 5. Introduction Continued 6. Aims 7. Aims Continued / Itinerary 8. Itinerary Continued / Objective / the Santiago Metropolitan Park 9. The Santiago Metropolitan Park Continued 10. The Santiago Metropolitan Park Continued 11. Jardín Botánico Chagual / Jardin Botanico Nacional, Viña del Mar 12. Jardin Botanico Nacional Viña del Mar Continued 13. Jardin Botanico Nacional Viña del Mar Continued 14. Jardin Botanico Nacional Viña del Mar Continued / La Campana National Park 15. La Campana National Park Continued / Huilo Huilo Biological Reserve Valdivian Temperate Rainforest 16. Huilo Huilo Biological Reserve Valdivian Temperate Rainforest Continued 17. Huilo Huilo Biological Reserve Valdivian Temperate Rainforest Continued 18. Huilo Huilo Biological Reserve Valdivian Temperate Rainforest Continued / Volcano Osorno 19. Volcano Osorno Continued / Vicente Perez Rosales National Park 20. Vicente Perez Rosales National Park Continued / Alerce Andino National Park 21. Alerce Andino National Park Continued 22. Francisco Coloane Marine Park 23. Francisco Coloane Marine Park Continued 24. Francisco Coloane Marine Park Continued / Outcomes 25. Expenditure / Thank you 2 Eliot Barden Kew Diploma Course 53 July 2017 Table of Figures Figure 1.) Valdivian Temperate Rainforest Alerce Andino [Photograph; Author] May (2017) Figure 2. Map of National parks of Chile Figure 3. Map of Chile Figure 4. Santiago Metropolitan Park [Photograph; Author] May (2017) Figure 5. -

Bosque Pehuén Park's Flora: a Contribution to the Knowledge of the Andean Montane Forests in the Araucanía Region, Chile Author(S): Daniela Mellado-Mansilla, Iván A
Bosque Pehuén Park's Flora: A Contribution to the Knowledge of the Andean Montane Forests in the Araucanía Region, Chile Author(s): Daniela Mellado-Mansilla, Iván A. Díaz, Javier Godoy-Güinao, Gabriel Ortega-Solís and Ricardo Moreno-Gonzalez Source: Natural Areas Journal, 38(4):298-311. Published By: Natural Areas Association https://doi.org/10.3375/043.038.0410 URL: http://www.bioone.org/doi/full/10.3375/043.038.0410 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. R E S E A R C H A R T I C L E ABSTRACT: In Chile, most protected areas are located in the southern Andes, in mountainous land- scapes at mid or high altitudes. Despite the increasing proportion of protected areas, few have detailed inventories of their biodiversity. This information is essential to define threats and develop long-term • integrated conservation programs to face the effects of global change. -

Nutrient Cycling in Nothofagus Pumilio Forests Along an Altitudinal Gradient in Tierra Del Fuego, Argentina Jorge L
Forest Ecology and Management 217 (2005) 80–94 www.elsevier.com/locate/foreco Nutrient cycling in Nothofagus pumilio forests along an altitudinal gradient in Tierra del Fuego, Argentina Jorge L. Frangi a, Marcelo D. Barrera a, Laura L. Richter a, Ariel E. Lugo b,* a Laboratorio de Investigacio´n de Sistemas Ecolo´gicos y Ambientales (LISEA), Universidad Nacional de La Plata. CC 31, 1900 La Plata, Argentina b International Institute of Tropical Forestry, USDA Forest Service, P.O. Box 25000, Rı´o Piedras, PR 00928-5000, USA Received 17 November 2004; received in revised form 20 May 2005; accepted 23 May 2005 Abstract Nothofagus pumilio (lenga) forests form monocultures from sea level to timberline in Tierra de Fuego, Argentina. Past studies suggested that the life form change from erect forest to krummholz had advantages to forest function. Aboveground net primary productivity (NPP) and organic matter production per unit leaf area and growing season day were higher in krummholz than in adjacent short erect forests at lower elevation. We compared tall erect, short erect, and krummholz lenga stands in terms of the concentration, accumulation, fluxes, turnover, and use-efficiency of nutrients (N, P, K, Ca, and Mg) along an elevation gradient (220–640 m) in Tierra del Fuego (Valle de Andorra, 54890S, 68820W). With few exceptions, patterns of decreasing values of nutrient concentration, nutrient stock, nutrient flux, and nutrient turnover reversed at the krummholz, which had higher values of these parameters than an adjacent short erect forest at lower elevation. Nutrient cycles accelerated at the krummholz but nutrient use-efficiency of organic matter production and nutrient return to the forest floor decreased. -

Survival and Growth of Nothofagus Pumilio Seedlings Under Several Microenvironments After Variable Retention Harvesting in Southern Patagonian Forests Guillermo J
Survival and growth of Nothofagus pumilio seedlings under several microenvironments after variable retention harvesting in southern Patagonian forests Guillermo J. Martínez Pastur, Rosina Soler Esteban, Juan Cellini, María V. Lencinas, Pablo L. Peri, Mark G. Neyland To cite this version: Guillermo J. Martínez Pastur, Rosina Soler Esteban, Juan Cellini, María V. Lencinas, Pablo L. Peri, et al.. Survival and growth of Nothofagus pumilio seedlings under several microenvironments after variable retention harvesting in southern Patagonian forests. Annals of Forest Science, Springer Nature (since 2011)/EDP Science (until 2010), 2014, 71 (3), pp.349 - 362. 10.1007/s13595-013-0343-3. hal- 01101533 HAL Id: hal-01101533 https://hal.archives-ouvertes.fr/hal-01101533 Submitted on 8 Jan 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annals of Forest Science (2014) 71:349–362 DOI 10.1007/s13595-013-0343-3 ORIGINAL PAPER Survival and growth of Nothofagus pumilio seedlings under several microenvironments after variable retention harvesting in southern Patagonian forests Guillermo J. Martínez Pastur & Rosina Soler Esteban & Juan M. Cellini & María V. Lencinas & Pablo L. Peri & Mark G. Neyland Received: 30 July 2013 /Accepted: 30 October 2013 /Published online: 22 November 2013 # INRA and Springer-Verlag France 2013 Abstract forest structure, microclimate, soil properties, and nutrient & Context Variable retention prescriptions for Nothofagus availability. -

Universidad De Chile Facultad De Ciencias Forestales Y De La
UNIVERSIDAD DE CHILE FACULTAD DE CIENCIAS FORESTALES Y DE LA CONSERVACION DE LA NATURALEZA ESCUELA DE CIENCIAS FORESTALES DEPARTAMENTO DE SILVICULTURA Y CONSERVACIÓN DE LA NATURALEZA COMPOSICIÓN FLORÍSTICA Y DIVERSIDAD DEL SOTOBOSQUE EN BOSQUES DE Nothofagus pumilio (Poepp et Endl.) Krasser DESPUÉS DEL RETROCESO DE LOS GLACIARES O’HIGGINS Y CHICO, CAMPO DE HIELO SUR Memoria para optar al Título Profesional de Ingeniera Forestal SOFÍA MARILYN OLIVARES FIGUEROA Profesor Guía: Sr. Álvaro Promis Baeza. Ingeniero Forestal, Doctor en Recursos Naturales Santiago, Chile 2017 UNIVERSIDAD DE CHILE FACULTAD DE CIENCIAS FORESTALES Y DE LA CONSERVACIÓN DE LA NATURALEZA ESCUELA DE CIENCIAS FORESTALES DEPARTAMENTO DE SILVICULTURA Y CONSERVACIÓN DE LA NATURALEZA COMPOSICIÓN FLORÍSTICA Y DIVERSIDAD DEL SOTOBOSQUE EN BOSQUES DE Nothofagus pumilio (Poepp et Endl.) Krasser DESPUÉS DEL RETROCESO DE LOS GLACIARES O’HIGGINS Y CHICO, CAMPO DE HIELO SUR Memoria para optar al Título Profesional de Ingeniera Forestal SOFÍA MARILYN OLIVARES FIGUEROA Calificaciones Nota Firma Prof. Guía Sr. Álvaro Promis 7,0 …………… Prof. Consejero Sr. Nicolás García 7,0 …………… Prof. Consejero Sr. Juan Pablo Fuentes 7,0 …………… AGRADECIMIENTOS Al profesor Álvaro Promis, por su compromiso y constancia para la realización de este trabajo. Por fomentar en mí el cuestionamiento, la observación y reflexión. Al Instituto Chileno de Campos de Hielo, por permitirme formar parte del programa Ciencia Joven y participar de la expedición que nos llevó a hasta uno de esos recónditos destinos, que cualquier amante y estudioso de la naturaleza quisiera visitar. A mis profesores consejeros Nicolás García y Juan Pablo Fuentes, por su disposición, contribución y acotaciones. A cada uno de las personas que dedicaron su tiempo de una u otra forma en la realización de este trabajo, escuchándome, aconsejándome, aclarando dudas, o apoyando directamente alguna de las actividades llevadas a cabo. -

Asteraceae) De Chile
Gayana Bot. 69(1): 9-29, 2012 ISSN 0016-5301 Actualización sistemática y distribución geográfica de Mutisioideae (Asteraceae) de Chile Systematic revision and geographic distribution of Chilean Mutisioideae (Asteraceae) ANDRÉS MOREIRA-MUÑOZ1, VANEZZA MORALES1 & MÉLICA MUÑOZ-SCHICK2 1Instituto de Geografía, Pontificia Universidad Católica de Chile, Vicuña Mackenna 4860, Macul, Santiago, Chile. 2Museo Nacional de Historia Natural, Casilla 787, Santiago, Chile. [email protected]; [email protected]; [email protected] RESUMEN Se presenta una actualización sistemática y de distribución geográfica de las especies y categorías infraespecíficas de la subfamilia Mutisioideae (tribus Mutisieae, Nassauvieae y Onoserideae) para Chile. El trabajo fue realizado sobre la base de bibliografía y la revisión de ejemplares principalmente de los herbarios SGO y CONC. Los resultados arrojan la presencia de 28 géneros, 192 especies y otros 22 taxones infraespecíficos en Chile. Siete de estos géneros, 77 especies y 12 taxones infraespecíficos tienen carácter de endémicos para el país. La revisión arroja dos adiciones y dos sustracciones a la flora de Chile. Adicionalmente, 47 de los taxones han sido corregidos en cuanto a su distribución geográfica por región en Chile. Se discute finalmente las implicancias que posee un adecuado conocimiento de la distribución geográfica de las especies para estudios de biogeografía y conservación de la flora nativa. PALABRAS CLAVE: Asteraceae, Chile, Compositae, diversidad, endemismo, Mutisieae, Nassauvieae, Onoserideae. ABSTRACT A systematic revision including the geographic distribution of the taxa pertaining to the Chilean Mutisioideae (tribes Mutisieae, Nassauvieae and Onoserideae) has been undertaken. The study has been done by means of the revision of available monographs and the most recent regional checklist, together with the examination of exemplars from SGO and CONC herbaria. -

Commonality and Variability in the Structural Attributes of Moist Temperate Old-Growth Forests: a Global Review ⇑ Sabina Burrascano A, William S
Forest Ecology and Management 291 (2013) 458–479 Contents lists available at SciVerse ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco Review Commonality and variability in the structural attributes of moist temperate old-growth forests: A global review ⇑ Sabina Burrascano a, William S. Keeton b, Francesco M. Sabatini a, , Carlo Blasi a a Department of Environmental Biology, Sapienza University of Rome, Rome, Italy b Rubenstein School of Environment and Natural Resources, University of Vermont, Burlington, VT 05405, USA article info abstract Article history: Temperate forests have been fundamentally altered by land use and other stressors globally; these have Received 3 August 2012 reduced the abundance of primary and old-growth forests in particular. Despite many regional studies, Received in revised form 15 October 2012 the literature lacks a global synthesis of temperate old-growth structural characteristics. In this study Accepted 18 November 2012 we compare literature derived data on mature and old-growth moist temperate forests with the aim of: (i) exploring global commonalities; (ii) investigating sources of variability among systems; and (iii) highlighting data gaps and research needs. We compiled a dataset of 147 records from 93 papers, and Keywords: analyzed a set of structural indicators: basal area, stem density, large living trees, live aboveground bio- Literature search mass, quadratic mean diameter, and coarse woody debris volume. These indicators were contrasted Forest dynamics Sustainable forest management between mature and old-growth age classes at a global level and across continents and broad forest Carbon sequestration types, testing for significance through Monte-Carlo permutation procedure. We also related structural Biodiversity indicators to age, climatic and geographical descriptors. -

9. a 10 Year Trial with South American Trees and Shrubs with Special
9. A 10 year trial with SouthAmerican trees and shrubswith specialregard to the Ir,lothofaglzsspp. I0 6ra royndir vid suduramerikonskumtroum og runnum vid serligumatliti at Nothofagw-slogum SarenOdum Abstract The potential of the ligneous flora of cool temperate South America in arboriculture in the Faroe Isles is elucidated through experimental planting of a broad variety of speciescollected on expeditions to Patagonia and Tierra del Fuego 1975 andl9T9.Particular good results have been obtained with the southernmost origins of Nothofagus antarctica, N. betuloides, and N. pumilio, of which a total of 6.500 plants were directly transplanted from Tierra del Fuego to the Faroe Isles in 1979. Soren Odum, Royal Vet.& Agric. IJniv., Arboretum, DK-2970 Horsholm, Denmark. Introduction As a student of botany at the University of CopenhagenI got the opportunity to get a job for the summer 1960as a member of the team mapping the flora of the Faroe Isles (Kjeld Hansen 1966). State geologist of the Faroe Isles and the Danish Geological Survey, J6annesRasmussen, provided working facilities for the team at the museum, and also my co-student,J6hannes J6hansen participated in the field. This stay and work founded my still growing interest in the Faroese nature and culture, and the initial connections between the Arboretum in Horsholm and Tbrshavn developed from this early contact with J6annesRasmussen and J6hannes J6hansen. On our way back to Copenhagen in 1960 onboard "Tjaldur", we called on Lerwick, Shetland, where I saw Hebe and Olearia in some gardens. This made it obvious to me, that if the Faroe Isles for historical reasonshad been more or less British rather than Nordic, the gardensof T6rshavn would, no doubt, have been speckledwith genera from the southern Hemisphere and with other speciesand cultivars nowadays common in Scottish nurseries and gardens.