Network-Based Approaches Elucidate Differences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Full Text (PDF)
Published OnlineFirst January 23, 2019; DOI: 10.1158/0008-5472.CAN-18-1261 Cancer Genome and Epigenome Research Sleeping Beauty Insertional Mutagenesis Reveals Important Genetic Drivers of Central Nervous System Embryonal Tumors Pauline J. Beckmann1, Jon D. Larson1, Alex T. Larsson1, Jason P. Ostergaard1, Sandra Wagner1, Eric P. Rahrmann1,2, Ghaidan A. Shamsan3, George M. Otto1,4, Rory L. Williams1,5, Jun Wang6, Catherine Lee6, Barbara R. Tschida1, Paramita Das1, Adrian M. Dubuc7, Branden S. Moriarity1, Daniel Picard8,9, Xiaochong Wu10, Fausto J. Rodriguez11, Quincy Rosemarie1,12, Ryan D. Krebs1, Amy M. Molan1,13, Addison M. Demer1, Michelle M. Frees1, Anthony E. Rizzardi14, Stephen C. Schmechel14,15, Charles G. Eberhart16, Robert B. Jenkins17, Robert J. Wechsler-Reya6, David J. Odde3, Annie Huang18, Michael D. Taylor10, Aaron L. Sarver1, and David A. Largaespada1 Abstract Medulloblastoma and central nervous system primitive identified several putative proto-oncogenes including Arh- neuroectodermal tumors (CNS-PNET) are aggressive, poorly gap36, Megf10,andFoxr2. Genetic manipulation of these differentiated brain tumors with limited effective therapies. genes demonstrated a robust impact on tumorigenesis Using Sleeping Beauty (SB) transposon mutagenesis, we in vitro and in vivo. We also determined that FOXR2 interacts identified novel genetic drivers of medulloblastoma and with N-MYC, increases C-MYC protein stability, and acti- CNS-PNET. Cross-species gene expression analyses classified vates FAK/SRC signaling. Altogether, our study identified SB-driven tumors into distinct medulloblastoma and several promising therapeutic targets in medulloblastoma CNS-PNET subgroups, indicating they resemble human and CNS-PNET. Sonic hedgehog and group 3 and 4 medulloblastoma and CNS neuroblastoma with FOXR2 activation. -

140503 IPF Signatures Supplement Withfigs Thorax
Supplementary material for Heterogeneous gene expression signatures correspond to distinct lung pathologies and biomarkers of disease severity in idiopathic pulmonary fibrosis Daryle J. DePianto1*, Sanjay Chandriani1⌘*, Alexander R. Abbas1, Guiquan Jia1, Elsa N. N’Diaye1, Patrick Caplazi1, Steven E. Kauder1, Sabyasachi Biswas1, Satyajit K. Karnik1#, Connie Ha1, Zora Modrusan1, Michael A. Matthay2, Jasleen Kukreja3, Harold R. Collard2, Jackson G. Egen1, Paul J. Wolters2§, and Joseph R. Arron1§ 1Genentech Research and Early Development, South San Francisco, CA 2Department of Medicine, University of California, San Francisco, CA 3Department of Surgery, University of California, San Francisco, CA ⌘Current address: Novartis Institutes for Biomedical Research, Emeryville, CA. #Current address: Gilead Sciences, Foster City, CA. *DJD and SC contributed equally to this manuscript §PJW and JRA co-directed this project Address correspondence to Paul J. Wolters, MD University of California, San Francisco Department of Medicine Box 0111 San Francisco, CA 94143-0111 [email protected] or Joseph R. Arron, MD, PhD Genentech, Inc. MS 231C 1 DNA Way South San Francisco, CA 94080 [email protected] 1 METHODS Human lung tissue samples Tissues were obtained at UCSF from clinical samples from IPF patients at the time of biopsy or lung transplantation. All patients were seen at UCSF and the diagnosis of IPF was established through multidisciplinary review of clinical, radiological, and pathological data according to criteria established by the consensus classification of the American Thoracic Society (ATS) and European Respiratory Society (ERS), Japanese Respiratory Society (JRS), and the Latin American Thoracic Association (ALAT) (ref. 5 in main text). Non-diseased normal lung tissues were procured from lungs not used by the Northern California Transplant Donor Network. -

1714 Gene Comprehensive Cancer Panel Enriched for Clinically Actionable Genes with Additional Biologically Relevant Genes 400-500X Average Coverage on Tumor
xO GENE PANEL 1714 gene comprehensive cancer panel enriched for clinically actionable genes with additional biologically relevant genes 400-500x average coverage on tumor Genes A-C Genes D-F Genes G-I Genes J-L AATK ATAD2B BTG1 CDH7 CREM DACH1 EPHA1 FES G6PC3 HGF IL18RAP JADE1 LMO1 ABCA1 ATF1 BTG2 CDK1 CRHR1 DACH2 EPHA2 FEV G6PD HIF1A IL1R1 JAK1 LMO2 ABCB1 ATM BTG3 CDK10 CRK DAXX EPHA3 FGF1 GAB1 HIF1AN IL1R2 JAK2 LMO7 ABCB11 ATR BTK CDK11A CRKL DBH EPHA4 FGF10 GAB2 HIST1H1E IL1RAP JAK3 LMTK2 ABCB4 ATRX BTRC CDK11B CRLF2 DCC EPHA5 FGF11 GABPA HIST1H3B IL20RA JARID2 LMTK3 ABCC1 AURKA BUB1 CDK12 CRTC1 DCUN1D1 EPHA6 FGF12 GALNT12 HIST1H4E IL20RB JAZF1 LPHN2 ABCC2 AURKB BUB1B CDK13 CRTC2 DCUN1D2 EPHA7 FGF13 GATA1 HLA-A IL21R JMJD1C LPHN3 ABCG1 AURKC BUB3 CDK14 CRTC3 DDB2 EPHA8 FGF14 GATA2 HLA-B IL22RA1 JMJD4 LPP ABCG2 AXIN1 C11orf30 CDK15 CSF1 DDIT3 EPHB1 FGF16 GATA3 HLF IL22RA2 JMJD6 LRP1B ABI1 AXIN2 CACNA1C CDK16 CSF1R DDR1 EPHB2 FGF17 GATA5 HLTF IL23R JMJD7 LRP5 ABL1 AXL CACNA1S CDK17 CSF2RA DDR2 EPHB3 FGF18 GATA6 HMGA1 IL2RA JMJD8 LRP6 ABL2 B2M CACNB2 CDK18 CSF2RB DDX3X EPHB4 FGF19 GDNF HMGA2 IL2RB JUN LRRK2 ACE BABAM1 CADM2 CDK19 CSF3R DDX5 EPHB6 FGF2 GFI1 HMGCR IL2RG JUNB LSM1 ACSL6 BACH1 CALR CDK2 CSK DDX6 EPOR FGF20 GFI1B HNF1A IL3 JUND LTK ACTA2 BACH2 CAMTA1 CDK20 CSNK1D DEK ERBB2 FGF21 GFRA4 HNF1B IL3RA JUP LYL1 ACTC1 BAG4 CAPRIN2 CDK3 CSNK1E DHFR ERBB3 FGF22 GGCX HNRNPA3 IL4R KAT2A LYN ACVR1 BAI3 CARD10 CDK4 CTCF DHH ERBB4 FGF23 GHR HOXA10 IL5RA KAT2B LZTR1 ACVR1B BAP1 CARD11 CDK5 CTCFL DIAPH1 ERCC1 FGF3 GID4 HOXA11 IL6R KAT5 ACVR2A -

Somatic Mutational Landscapes of Adherens Junctions and Their
1 Somatic mutational landscapes of adherens junctions and their 2 functional consequences in cutaneous melanoma development 3 4 Praveen Kumar Korla,1 Chih-Chieh Chen,2 Daniel Esguerra Gracilla,1 Ming-Tsung Lai,3 Chih- 5 Mei Chen,4 Huan Yuan Chen,5 Tritium Hwang,1 Shih-Yin Chen,4,6,* Jim Jinn-Chyuan Sheu1,4, 6-9,* 6 1Institute of Biomedical Sciences, National Sun Yat-sen University, Kaohsiung 80242, Taiwan; 7 2Institute of Medical Science and Technology, National Sun Yat-sen University, Kaohsiung 80424, 8 Taiwan; 3Department of Pathology, Taichung Hospital, Ministry of Health and Welfare, Taichung 9 40343, Taiwan; 4Genetics Center, China Medical University Hospital, Taichung 40447, Taiwan; 10 5Institute of Biomedical Sciences, Academia Sinica, Taipei 11574, Taiwan; 6School of Chinese 11 Medicine, China Medical University, Taichung 40402, Taiwan; 7Department of Health and 12 Nutrition Biotechnology, Asia University, Taichung 41354, Taiwan; 8Department of 13 Biotechnology, Kaohsiung Medical University, Kaohsiung 80708, Taiwan; 9Institute of 14 Biopharmaceutical Sciences, National Sun Yat-sen University, Kaohsiung 80242, Taiwan 15 16 PKK, CCC and DEG contributed equally to this study. 17 *Correspondence to: Dr. Shih-Yin Chen ([email protected]) at Genetics Center, China 18 Medical University Hospital, Taichung, 40447, TAIWAN; or Dr. Jim Jinn-Chyuan Sheu 19 ([email protected]) at Institute of Biomedical Sciences, National Sun Yat-sen 20 University, Kaohsiung City 80424, TAIWAN. 21 22 Running title: mutational landscape of cadherins in melanoma 1 23 Abstract 24 Cell-cell interaction in skin homeostasis is tightly controlled by adherens junctions (AJs). 25 Alterations in such regulation lead to melanoma development. -

Sterculic Acid Alters Adhesion Molecules Expression and Extracellular Matrix Compounds to Regulate Migration of Lung Cancer Cells
cancers Article Sterculic Acid Alters Adhesion Molecules Expression and Extracellular Matrix Compounds to Regulate Migration of Lung Cancer Cells Rafael Peláez * , Rodrigo Ochoa, Ana Pariente, Ángela Villanueva-Martínez, Álvaro Pérez-Sala and Ignacio M. Larráyoz * Biomarkers and Molecular Signaling Group, Neurodegeneration Area, Center for Biomedical Research of La Rioja (CIBIR), Piqueras 98, 26006 Logroño, Spain; [email protected] (R.O.); [email protected] (A.P.); [email protected] (Á.V.-M.); [email protected] (Á.P.-S.) * Correspondence: [email protected] (R.P.); [email protected] (I.M.L.); Tel.: +34-941-278-770 ((ext. 84866) (R.P.) & (ext. 89878) (I.M.L.)) Simple Summary: Sterculic acid (SA) is a naturally occurring lipid with SCD1 inhibitory activity, but it also modifies many other pathways and underlying gene expression. SCD upregulation has been associated with tumor aggressiveness and progression. Effects of SA treatment over extracellular matrix compounds and adhesion molecule expression have not been described in cancer cells up to now. Our results show that SA induces cell death at high dose, but we also observed that lower concentrations of SA treatments also reduce cell adhesion-migration and modify integrins and extracellular matrix compounds expression. Citation: Peláez, R.; Ochoa, R.; Abstract: Sterculic acid (SA) is a cyclopropenoid fatty acid isolated from Sterculia foetida seeds. Pariente, A.; Villanueva-Martínez, Á.; This molecule is a well-known inhibitor of SCD1 enzyme, also known as D9-desaturase, which Pérez-Sala, Á.; Larráyoz, I.M. main function is related to lipid metabolism. However, recent studies have demonstrated that Sterculic Acid Alters Adhesion it also modifies many other pathways and the underlying gene expression. -

Pathway and Network Analysis of Somatic Mutations Across Cancer
Network Analysis of Mutaons Across Cancer Types Ben Raphael Fabio Vandin, Max Leiserson, Hsin-Ta Wu Department of Computer Science Center for Computaonal Molecular Biology Significantly Mutated Genes Muta#on Matrix Stascal test Genes Paents Frequency Number Paents Study Num. Samples Num. SMG TCGA Ovarian (2011) 316 10 TCGA Breast (2012) 510 35 TCGA Colorectal (2012) 276 32 background mutaon rate (BMR), gene specific effects, etc. Significantly Mutated Genes à Pathways Stascal test Frequency Number Paents TCGA Colorectal (Nature 2012) TCGA Ovarian (Nature 2011) background mutaon rate (BMR), gene specific effects, etc. Advantages of Large Datasets Prior knowledge of groups of genes Genes Paents Known pathways Interac3on Network None Prior knowledge • Novel pathways or interac3ons between pathways (crosstalk) • Topology of interac3ons Two Algorithms Prior knowledge of groups of genes Genes Paents Known pathways Interac3on Network None Prior knowledge Number of Hypotheses HotNet subnetworks of Dendrix interac3on network Exclusive gene sets HotNet: Problem Defini3on Given: 1. Network G = (V, E) V = genes. E = interac3ons b/w genes 2. Binary mutaon matrix Genes = mutated = not mutated Paents Find: Connected subnetworks mutated in a significant number of paents. Subnetwork Properes Mutaon frequency/score AND network topology Frequency Number Paents • Moderate frequency/score • High frequency/score • Highly connected • Connected through high-degree node. Example: TP53 has 238 neighbors in HPRD network Mutated subnetworks: HotNet* Muta#on Matrix Human Interac#on Network Genes = mutated genes Paents (1) Muta#on à heat diffusion Extract “significantly hot” subnetworks Hot (2) Cold *F. Vandin, E. Upfal, and B. J. Raphael. J. Comp.Biol. (2011). Also RECOMB (2010). Stas3cal Test Muta#on Matrix Random Binary Matrix Genes Genes Paents Paents Xs = number of subnetworks ≥ s genes Two-stage mul-hypothesis test: Rigorously bound FDR. -
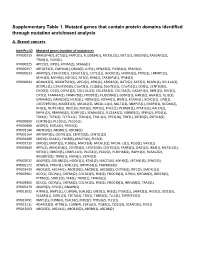
Supplementary Table 1. Mutated Genes That Contain Protein Domains Identified Through Mutation Enrichment Analysis
Supplementary Table 1. Mutated genes that contain protein domains identified through mutation enrichment analysis A. Breast cancers InterPro ID Mutated genes (number of mutations) IPR000219 ARHGEF4(2), ECT2(1), FARP1(1), FLJ20184(1), MCF2L2(1), NET1(1), OBSCN(5), RASGRF2(2), TRAD(1), VAV3(1) IPR000225 APC2(2), JUP(1), KPNA5(2), SPAG6(1) IPR000357 ARFGEF2(2), CMYA4(1), DRIM(2), JUP(1), KPNA5(2), PIK3R4(1), SPAG6(1) IPR000533 AKAP9(2), C10orf39(1), C20orf23(1), CUTL1(1), HOOK1(1), HOOK3(1), KTN1(2), LRRFIP1(3), MYH1(3), MYH9(2), NEF3(1), NF2(1), RSN(1), TAX1BP1(1), TPM4(1) IPR000694 ADAM12(3), ADAMTS19(1), APC2(2), APXL(1), ARID1B(1), BAT2(2), BAT3(1), BCAR1(1), BCL11A(2), BCORL1(1), C14orf155(3), C1orf2(1), C1QB(1), C6orf31(1), C7orf11(1), CD2(1), CENTD3(3), CHD5(3), CIC(3), CMYA1(2), COL11A1(3), COL19A1(2), COL7A1(3), DAZAP1(1), DBN1(3), DVL3(1), EIF5(1), FAM44A(1), FAM47B(1), FHOD1(1), FLJ20584(1), G3BP2(2), GAB1(2), GGA3(1), GLI1(3), GPNMB(2), GRIN2D(3), HCN3(1), HOXA3(2), HOXA4(1), IRS4(1), KCNA5(1), KCNC2(1), LIP8(1), LOC374955(1), MAGEE1(2), MICAL1(2), MICAL‐L1(1), MLLT2(1), MMP15(1), N4BP2(1), NCOA6(2), NHS(1), NUP214(3), ODZ1(3), PER1(2), PER2(1), PHC1(1), PLXNB1(1), PPM1E(2), RAI17(2), RAPH1(2), RBAF600(2), SCARF2(1), SEMA4G(1), SLC16A2(1), SORBS1(1), SPEN(2), SPG4(1), TBX1(1), TCF1(2), TCF7L1(1), TESK1(1), THG‐1(1), TP53(18), TRIF(1), ZBTB3(2), ZNF318(2) IPR000909 CENTB1(2), PLCB1(1), PLCG1(1) IPR000998 AEGP(3), EGFL6(2), PRSS7(1) IPR001140 ABCB10(2), ABCB6(1), ABCB8(2) IPR001164 ARFGAP3(1), CENTB1(2), CENTD3(3), CENTG1(2) IPR001589 -

Supplementary Table 3 Gene Microarray Analysis: PRL+E2 Vs
Supplementary Table 3 Gene microarray analysis: PRL+E2 vs. control ID1 Field1 ID Symbol Name M Fold P Value 69 15562 206115_at EGR3 early growth response 3 2,36 5,13 4,51E-06 56 41486 232231_at RUNX2 runt-related transcription factor 2 2,01 4,02 6,78E-07 41 36660 227404_s_at EGR1 early growth response 1 1,99 3,97 2,20E-04 396 54249 36711_at MAFF v-maf musculoaponeurotic fibrosarcoma oncogene homolog F 1,92 3,79 7,54E-04 (avian) 42 13670 204222_s_at GLIPR1 GLI pathogenesis-related 1 (glioma) 1,91 3,76 2,20E-04 65 11080 201631_s_at IER3 immediate early response 3 1,81 3,50 3,50E-06 101 36952 227697_at SOCS3 suppressor of cytokine signaling 3 1,76 3,38 4,71E-05 16 15514 206067_s_at WT1 Wilms tumor 1 1,74 3,34 1,87E-04 171 47873 238623_at NA NA 1,72 3,30 1,10E-04 600 14687 205239_at AREG amphiregulin (schwannoma-derived growth factor) 1,71 3,26 1,51E-03 256 36997 227742_at CLIC6 chloride intracellular channel 6 1,69 3,23 3,52E-04 14 15038 205590_at RASGRP1 RAS guanyl releasing protein 1 (calcium and DAG-regulated) 1,68 3,20 1,87E-04 55 33237 223961_s_at CISH cytokine inducible SH2-containing protein 1,67 3,19 6,49E-07 78 32152 222872_x_at OBFC2A oligonucleotide/oligosaccharide-binding fold containing 2A 1,66 3,15 1,23E-05 1969 32201 222921_s_at HEY2 hairy/enhancer-of-split related with YRPW motif 2 1,64 3,12 1,78E-02 122 13463 204015_s_at DUSP4 dual specificity phosphatase 4 1,61 3,06 5,97E-05 173 36466 227210_at NA NA 1,60 3,04 1,10E-04 117 40525 231270_at CA13 carbonic anhydrase XIII 1,59 3,02 5,62E-05 81 42339 233085_s_at OBFC2A oligonucleotide/oligosaccharide-binding -

The Consensus Coding Sequences of Human Breast and Colorectal Cancers Tobias Sjöblom,1* Siân Jones,1* Laura D
The Consensus Coding Sequences of Human Breast and Colorectal Cancers Tobias Sjöblom,1* Siân Jones,1* Laura D. Wood,1* D. Williams Parsons,1* Jimmy Lin,1 Thomas Barber,1 Diana Mandelker,1 Rebecca J. Leary,1 Janine Ptak,1 Natalie Silliman,1 Steve Szabo,1 Phillip Buckhaults,2 Christopher Farrell,2 Paul Meeh,2 Sanford D. Markowitz,3 Joseph Willis,4 Dawn Dawson,4 James K. V. Willson,5 Adi F. Gazdar,6 James Hartigan,7 Leo Wu,8 Changsheng Liu,8 Giovanni Parmigiani,9 Ben Ho Park,10 Kurtis E. Bachman,11 Nickolas Papadopoulos,1 Bert Vogelstein,1† Kenneth W. Kinzler,1† Victor E. Velculescu1† 1Ludwig Center and Howard Hughes Medical Institute, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD 21231, USA. 2Department of Pathology and Microbiology, Center for Colon Cancer Research, and South Carolina Cancer Center, Division of Basic Research, University of South Carolina School of Medicine, Columbia, SC 29229, USA. 3Department of Medicine, Ireland Cancer Center, and Howard Hughes Medical Institute, Case Western Reserve University and University Hospitals of Cleveland, Cleveland, OH 44106, USA. 4Department of Pathology and Ireland Cancer Center, Case Western Reserve University and University Hospitals of Cleveland, Cleveland, OH 44106, USA. 5Harold C. Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA. 6Hamon Center for Therapeutic Oncology Research and Department of Pathology, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA. 7Agencourt Bioscience Corporation, Beverly, MA 01915, USA. 8SoftGenetics LLC, State College, PA 16803, USA. 9Departments of Oncology, Biostatistics, and Pathology, Johns Hopkins Medical Institutions, Baltimore, MD 21205, USA. -

Fibroblasts from the Human Skin Dermo-Hypodermal Junction Are
cells Article Fibroblasts from the Human Skin Dermo-Hypodermal Junction are Distinct from Dermal Papillary and Reticular Fibroblasts and from Mesenchymal Stem Cells and Exhibit a Specific Molecular Profile Related to Extracellular Matrix Organization and Modeling Valérie Haydont 1,*, Véronique Neiveyans 1, Philippe Perez 1, Élodie Busson 2, 2 1, 3,4,5,6, , Jean-Jacques Lataillade , Daniel Asselineau y and Nicolas O. Fortunel y * 1 Advanced Research, L’Oréal Research and Innovation, 93600 Aulnay-sous-Bois, France; [email protected] (V.N.); [email protected] (P.P.); [email protected] (D.A.) 2 Department of Medical and Surgical Assistance to the Armed Forces, French Forces Biomedical Research Institute (IRBA), 91223 CEDEX Brétigny sur Orge, France; [email protected] (É.B.); [email protected] (J.-J.L.) 3 Laboratoire de Génomique et Radiobiologie de la Kératinopoïèse, Institut de Biologie François Jacob, CEA/DRF/IRCM, 91000 Evry, France 4 INSERM U967, 92260 Fontenay-aux-Roses, France 5 Université Paris-Diderot, 75013 Paris 7, France 6 Université Paris-Saclay, 78140 Paris 11, France * Correspondence: [email protected] (V.H.); [email protected] (N.O.F.); Tel.: +33-1-48-68-96-00 (V.H.); +33-1-60-87-34-92 or +33-1-60-87-34-98 (N.O.F.) These authors contributed equally to the work. y Received: 15 December 2019; Accepted: 24 January 2020; Published: 5 February 2020 Abstract: Human skin dermis contains fibroblast subpopulations in which characterization is crucial due to their roles in extracellular matrix (ECM) biology. -

The Genetics of Malignant Melanoma Jesus Lomas1, Pilar Martin-Duque2
The genetics of malignant melanoma Jesus Lomas1, Pilar Martin-Duque2, Mar Pons1, Miguel Quintanilla1 1Instituto de Investigaciones Biomedicas Alberto Sols, Consejo Superior de Investigaciones Cientificas-Universidad Autonoma de Madrid, and 2Facultad de Ciencias Biosanitaria, Universidad Francisco de Vitoria (PM-D), Madrid, Spain TABLE OF CONTENTS 1. Abstract 2. Introduction 3. Susceptibility genes 3.1. The CDKN2A locus and familial melanoma 3.2. MSH/MC1R and the regulation of pigmentation 4. Genetic alterations found in sporadic melanoma 4.1. Receptor tyrosine kinases 4.2. RAF, RAS, and the mitogen-activated protein kinase (MAPK) pathway 4.3. PTEN and the phosphatidylinositol 3’-kinase (PI3K) pathway 4.4. Microphthalmia-associated transcriptional factor 4.5. The Wnt/beta-catenin signaling pathway 5. Genetically engineered animal models of melanoma 6. Genomic and gene-expression profiling studies 6.1. Gene expression profiles 6.2. Comparative genomic hybridization 7. Melanoma stem cells 8. Conclusions and perspectives 9. Acknowledgements 10. References 1. ABSTRACT 2. INTRODUCTION Melanoma probably is the most aggressive cancer in humans and remains one of the leading causes of cancer The incidence of melanoma is rising by 3-8% per death in developed countries. This review summarizes the year in the Caucasian population (1). This fact together most important alterations in protooncogenes and tumor with the lack of remarkable therapeutic improvements suppressor genes that contribute to the pathogenesis of represents a relevant health problem