Understanding the Fertilization and Irrigation Response of Seed Orchard Loblolly Pine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Botany for the Herbalist Common Plant Families 7Song, Director Northeast School of Botanical Medicine 7Song.Com
Botany for the Herbalist Common Plant Families 7Song, Director Northeast School of Botanical Medicine 7Song.com This handout describes common plant families in the US and Canada. Each family segment contains general characteristics of the family as well as some of the more commonly known species within. In some families such as the Liliaceae, the genera of the plants are still in transition and being placed in newly created families. In other families such as the Scrophulariaceae, some of the former genera have been placed into different already existing families. Within this handout they are generally kept in the older family grouping with the new family identified next to the genus. Field botany is the study of identifying plants and grouping them into categories based on shared anatomical characteristics. Much of this is based on their reproductive parts, especially their flowers. One of the most useful starting points to identify specific plants in the field is by knowing their plant families. Families are a useful category to understand, as plants within them may share obvious traits both botanical (anatomical similarities) and other qualities such as aromatics. As far as medicinal characteristics within a family, there are generally more exceptions to shared traits than similarities in uses. An example showing both sides this is the Apiaceae. This family includes many aromatic, flavorful plants such as Dill, Fennel, and Angelica but also Poison hemlock (Conium), a notoriously poisonous plant. Another example is the Liliaceae with edible foods such as garlic, onion and asparagus but also the toxic Veratrum and Lily-of-the-valley (Convallaria). -

Section 1. Western White Pine (Pinus Monticola)
40 - PART 1. CONSENSUS DOCUMENTS ON BIOLOGY OF TREES Section 1. Western white pine (Pinus monticola) 1. Taxonomy The largest genus in the family Pinaceae, Pinus L., which consists of about 110 pine species, occurs naturally through much of the Northern Hemisphere, from the far north to the cooler montane tropics (Peterson, 1980; Richardson, 1998). Two subgenera are usually recognised: hard pines (generally with much resin, wood close-grained, leaf fascicle sheath persistent, two fibrovascular bundles per needle — the diploxylon pines); and soft, or white pines (generally little resin, wood coarse-grained, sheath sheds early, one fibrovascular bundle in a needle — the haploxylon pines). These subgenera are called respectively subgenus Pinus and subgenus Strobus (Little and Critchfield, 1969; Price et al., 1998; Gernandt et al., 2005). Occasionally, one to about half the species (20 spp.) in subgenus Strobus have been classified instead in a variable subgenus Ducampopinus. Western white pine (Pinus monticola Dougl. ex D. Don) belongs to subgenus Strobus (Syring et al., 2007). Pinus monticola was classified by Critchfield and Little (1966) as one of 14 white pines in section Strobus, subsection Strobi, now call section Ouinquefoliae and subsection Strobus, respectively. Earlier classifications have varied in the number of species assigned to subsection Strobus, but P. monticola has consistently been grouped with the New World species P. ayacahuite, P. lambertiana, and P. strobus and the Old World species P. wallichiana (synonym P. griffithii) and P. peuce (Critchfield, 1986). A molecular phylogeny of the genus Pinus, based on the nuclear ribosomal DNA internal transcribed spacer (nrITS), did not support separation of subsection Strobus from either subsection Cembrae or subsection Krempfianae (Liston et al., 1999). -

Botany of Trees 2
Botany of Trees California’s Part 2: How Wood Forms Iconic Flora North Coast California Na.ve Plant Society Free Webinar 7 PM Tomorrow Night Dr. Ma9 Ri9er h9ps://us04web.zoom.us/webinar/register/ WN_s4i_BmnlTryUe_7t45a9Qw Botany of Trees Tuesday, April 14th: IntroducTon to trees, growth, development, leaves, and morphology • Five part series -Tuesdays, 11 AM to 12:30 PM • Each will be ~1 hour with 30 minutes of ques.ons Tuesday, April 28th: Tree names, Tuesday, April 21st: Trunks, branches, diversity, and why names change tree form, branching pa9erns, and shape, and how wood forms Tuesday, May 5th: Water in trees, Tuesday, May 12th: ReproducTon, photosynthesis, and respiraTon flower formaTon, fruit, and seeds All aboveground plant structures are: Interpret the sharp structure: stems, leaves, or buds Modified leaf - spine bud becoming a new branch Pointy Structures on Plants Big Leaf Maple (Acer macrophyllum) Thorn Spine Prickle modified branch modified leaf epidermal that comes from that comes from outgrowths that an axillary bud below the occur at random on axillary bud the stem (not necessarily at nodes) Big Leaf Maple (Acer Pine Leaves macrophyllum) • Leaves of two kinds: primary scales and secondary needles • Primary leaves of pines are membranous scales • A set number of needle leaves are produced on short branches (fascicles) • Each bundle (fascicle) of needles is surrounded by membranous bud scales Leaf Leaf Leaf Stump SprouTng & Epicormic Growth Meristems: where growth occurs • Apical Meristems • Form primary .ssues • Increase in -
1996 Elementary Envirothon
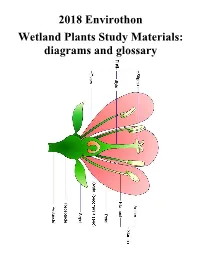
2018 Envirothon Wetland Plants Study Materials: diagrams and glossary Layers in a Deciduous Forest Ground Layer – leaf litter, fallen branches, lichens, clubmosses, and true mosses Herb Layer – short plants like ferns and trillium Shrub Layer – shrubs like rhododendrons, azaleas, mountain laurels, and huckleberries Understory (sapling on small tree layer) – short tree species (dogwood, sassafras) and young trees Canopy (tree layer) – the tallest layer, 60 -100 feet high, with large oak, maple, beech, hickory, elm, basswood, linden, walnut, white pine, hemlock, cedar GLOSSARY alternate The arrangement of leaves along a stem where consecutive leaves alternate along different sides of the stem. Taken together, all of the leaves plus the stem lie in roughly the same geometric plane. In winter (after leaf drop for deciduous plants), the arrangement can be determined by looking at the leaf scars or buds on the dormant twigs. axil The area between a leaf and stem, where an axillary bud forms. The axillary bud can be a floral bud that becomes a flower, or a vegetative bud that may produce a lateral stem (or remain dormant). basal Located at or near the base of a plant stem, or at the base of any other plant part: bole Another term for the trunk of a tree bud The tightly wrapped structure that contains miniaturized forms of leaves and stems (vegetative bud) which may open at a later date or remain dormant forever, or flowers (floral bud) which open the following spring. bundle The grouping together of needles on a pine tree into a unified cluster, bound by a sheath (or fascicle) at the base, and attached at a single point to the twig. -

Anatomy of Flowering Plants
This page intentionally left blank Anatomy of Flowering Plants Understanding plant anatomy is not only fundamental to the study of plant systematics and palaeobotany, but is also an essential part of evolutionary biology, physiology, ecology, and the rapidly expanding science of developmental genetics. In the third edition of her successful textbook, Paula Rudall provides a comprehensive yet succinct introduction to the anatomy of flowering plants. Thoroughly revised and updated throughout, the book covers all aspects of comparative plant structure and development, arranged in a series of chapters on the stem, root, leaf, flower, seed and fruit. Internal structures are described using magnification aids from the simple hand-lens to the electron microscope. Numerous references to recent topical literature are included, and new illustrations reflect a wide range of flowering plant species. The phylogenetic context of plant names has also been updated as a result of improved understanding of the relationships among flowering plants. This clearly written text is ideal for students studying a wide range of courses in botany and plant science, and is also an excellent resource for professional and amateur horticulturists. Paula Rudall is Head of Micromorphology(Plant Anatomy and Palynology) at the Royal Botanic Gardens, Kew. She has published more than 150 peer-reviewed papers, using comparative floral and pollen morphology, anatomy and embryology to explore evolution across seed plants. Anatomy of Flowering Plants An Introduction to Structure and Development PAULA J. RUDALL CAMBRIDGE UNIVERSITY PRESS Cambridge, New York, Melbourne, Madrid, Cape Town, Singapore, São Paulo Cambridge University Press The Edinburgh Building, Cambridge CB2 8RU, UK Published in the United States of America by Cambridge University Press, New York www.cambridge.org Information on this title: www.cambridge.org/9780521692458 © Paula J. -
Ornamental Garden Plants of the Guianas, Part 4
Bromeliaceae Epiphytic or terrestrial. Roots usually present as holdfasts. Leaves spirally arranged, often in a basal rosette or fasciculate, simple, sheathing at the base, entire or spinose- serrate, scaly-lepidote. Inflorescence terminal or lateral, simple or compound, a spike, raceme, panicle, capitulum, or a solitary flower; inflorescence-bracts and flower-bracts usually conspicuous, highly colored. Flowers regular (actinomorphic), mostly bisexual. Sepals 3, free or united. Petals 3, free or united; corolla with or without 2 scale-appendages inside at base. Stamens 6; filaments free, monadelphous, or adnate to corolla. Ovary superior to inferior. Fruit a dry capsule or fleshy berry; sometimes a syncarp (Ananas ). Seeds naked, winged, or comose. Literature: GENERAL: Duval, L. 1990. The Bromeliads. 154 pp. Pacifica, California: Big Bridge Press. Kramer, J. 1965. Bromeliads, The Colorful House Plants. 113 pp. Princeton, New Jersey: D. Van Nostrand Company. Kramer, J. 1981. Bromeliads.179pp. New York: Harper & Row. Padilla, V. 1971. Bromeliads. 134 pp. New York: Crown Publishers. Rauh, W. 1919.Bromeliads for Home, Garden and Greenhouse. 431pp. Poole, Dorset: Blandford Press. Singer, W. 1963. Bromeliads. Garden Journal 13(1): 8-12; 13(2): 57-62; 13(3): 104-108; 13(4): 146- 150. Smith, L.B. and R.J. Downs. 1974. Flora Neotropica, Monograph No.14 (Bromeliaceae): Part 1 (Pitcairnioideae), pp.1-658, New York: Hafner Press; Part 2 (Tillandsioideae), pp.663-1492, New York: Hafner Press; Part 3 (Bromelioideae), pp.1493-2142, Bronx, New York: New York Botanical Garden. Weber, W. 1981. Introduction to the taxonomy of the Bromeliaceae. Journal of the Bromeliad Society 31(1): 11-17; 31(2): 70-75. -

Identification Key for Coniferous Trees in Maryland
Identification Key for Coniferous Trees in Maryland By: Kerry Wixted & Madeline Koenig January 2017 Common Coniferous Tree Guide Key 1. Leaves scale-like……………………………………….…………pg 1 1. Leaves needles……………………………………………………2 2. Leaves in clusters/bundles…………………………….………..3 2. Leaves not in clusters/bundles………………………………....4 3. Leaves in clusters of 2……………………………………………pg 2 3. Leaves in clusters of 2-3……………………………….…………pg 3 3. Leaves clusters of 3…………………………………………….…pg 4 3. Leaves in clusters of 5………………………………………....…pg 6 4. Needles four-sided, stiff…………………………………...……pg 7 4. Needles flat, flexible & blunt………………………………..…pg 8 Larry Hogan, Governor Mark Belton, Secretary dnr.maryland.gov/wildlife i How To Use This Guide This guide was created as an easy-to-use reference for beginner identification. It is not a comprehensive guide for all conifers found in Maryland. Trees represented in the guide include those that are native to Maryland, those that are introduced (exotic), and those that are exotic and create ecological problems (invasive). To use this guide, begin with the key on the previous page. Read the statements and choose the statement that best matches the specimen you are viewing. Proceed to the next statement that matches the specimen you are viewing. Eventually, the key will lead you to the identification. Terms used in the key are illustrated on pages ii - iii. Photographs and images selected for this guide are intended to represent commonly found phenotypes. Images are not to scale. The following information will be listed for each species: • Common Name (Scientific name) • Leaf Description • Cone Description • Bark Description • Description of Overall Form Common Identification Terms: Scale-like Versus Needle Scale-like and needle refer to the shape of the leaf. -

Temperature and Genotype Influence Sweet Cherry
TEMPERATURE AND GENOTYPE INFLUENCE SWEET CHERRY POLLINATION BIOLOGY By LU ZHANG A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY Department of Horticulture DECEMBER 2014 To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of LU ZHANG find it satisfactory and recommend that it be accepted. ___________________________________ Matthew D. Whiting, Ph.D., Chair ___________________________________ Bhaskar Bondada, Ph.D. ___________________________________ Preston K. Andrews, Ph.D. ___________________________________ Roy A. Navarre, Ph.D. ii ACKNOWLEDGEMENTS I would like to express the deepest appreciation to my committee chair, Dr. Matthew Whiting, who showed me the road and always supported me on the path to this degree. Without his encouragement, understanding and patient help this dissertation would not have been possible. I am also thankful for the excellent example he has provided as a kind, bright and enthusiastic professor, and his personality charisma will inspire me to become a better person. I would also like to express my gratitude to my committee members, Dr. Roy Navarre, Dr. Preston Andrews and Dr. Bhaskar Bondada for their support, enthusiastic encouragement and invaluable advice for my research. I would like to express my special thanks to Dr. Caixi Zhang, Mr. Lynn E. Long and Dr. Yiannis Ampatzidis for their efforts and help on this work. I would also like to thank my friends and everyone in my lab for their help and support in my research and life. Special thanks to Ms. Laura Wells for valuable assistance and care. Finally, but most importantly, I am deeply thankful to my parents for their supporting and understanding. -

Western White Pine Management Conference
Proceedings of the 3rd Western White Pine Management Conference Best Western Vernon Lodge Vernon, British Columbia June 17-18, 2008 Edited by: Michelle R. Cleary BC Ministry of Forests and Range Southern Interior Forest Region Kamloops, British Columbia 1 Proceedings of the 3rd Western White Pine Management Conference Compiled and edited by: Michelle R. Cleary BC Ministry of Forests and Range Southern Interior Forest Region Kamloops, British Columbia 2010 This report is available in PDF format at http://www.for.gov.bc.ca/rsi/ForestHealth/Info_Reports.htm 2 Organizing Committee: Michelle Cleary, Forest Pathologist BC Ministry of Forests and Range, Southern Interior Forest Region Stefan Zeglen, Forest Pathologist BC Ministry of Forests and Range, Coast Forest Region Michael Carlson, Research Scientist BC Ministry of Forests and Range, Research Branch, Kalamalka Forestry Centre Diane Douglas BC Ministry of Forests and Range, Tree Improvement Branch Vicky Berger, Research Technician BC Ministry of Forests, Research Branch, Kalamalka Forestry Centre Acknowledgements: I would like to thank the authors for contributing to the proceedings and for their cooperation and effort in providing high-quality content in a manageable form. Many thanks to Kevin Buxton for his assistance. The following organizations are also thanked for their contributions and support... BC Ministry of Forests, Southern Interior Forest Region BC Ministry of Forests, Coast Forest Region BC Ministry of Forest, Research Branch BC Ministry of Forests, Tree Improvement Branch Forest Genetics Council of BC, ETAC BC Ministry of Forests, Okanagan Shuswap Forest District Allan Brooks Nature Centre Vernon Seed Orchard Company Select Seed Co. Ltd. Photo Credits: Neil MacEachern, Diane Douglas, Vicky Berger, and Forrest Joy For citation, please reference as follows: Author(s). -

Effects of Pinus Taeda Leaf Anatomy on Vascular and Extravascular Leaf
Effects of Pinus taeda leaf anatomy on vascular and extravascular leaf hydraulic conductance as influenced by N-fertilization and elevated CO2 Jean-Christophe Domec, Sari Palmroth, Ram Oren To cite this version: Jean-Christophe Domec, Sari Palmroth, Ram Oren. Effects of Pinus taeda leaf anatomy on vascu- lar and extravascular leaf hydraulic conductance as influenced by N-fertilization and elevated CO2. Journal of Plant Hydraulics, INRA Science and Impact, 2016, 3, pp.e007. 10.20870/jph.2016.e007. hal-01438773 HAL Id: hal-01438773 https://hal.archives-ouvertes.fr/hal-01438773 Submitted on 18 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Journal of Plant Hydraulics 3: e-007 Effects of Pinus taeda leaf anatomy on vascular and extravascular leaf hydraulic conductance as influenced by N-fertilization and elevated CO2 Jean-Christophe Domec1,2, Sari Palmroth2, and Ram Oren2 1Bordeaux Sciences Agro, UMR 1391 INRA-ISPA, 33175 Gradignan Cedex, France; 2Nicholas School of the Environment, Box 90328, Duke University, Durham, NC 27708, USA Corresponding author: Jean-Christophe Domec, [email protected] Date of submission: December18, 2015 Date of publication: March 26, 2015 Abstract Silvicultural practices (e.g., nitrogen addition through fertilization) and environmental changes (e.g., elevated [CO2]) may alter needle structure, impacting mass and energy exchange between the biosphere and atmosphere through alteration of stomatal function. -

Glossary - Botany-1
1 Glossary - Botany-1 Aleuron: (Gk. aleuron, flour) A proteinous material usually in the form of small granules occurring in the outermost cell layer of the endoderm of wheat and other grains; if contacted with water release a plant hormone triggering germination of the seed. Bark: The ruptured original cortex and epidermal layer of the seedling when secondary growth takes place; it consists mainly of periderm (underlying phloem and many layers of cork-cells that die but remain in place to make the outer protective bark). Cambium: (L. cambiare, to exchange) A meristem that gives rise to parallel rows of cells (secondary growth - dilatation); commonly applied to the vascular cambium and to the cork cambium, or phellogen. Cork C.: The outer part of the lateral meristem that forms the periderm, producing cork (phellem) and growth rings toward the surface (outside) of the plant and phelloderm toward the inside; common in stems and roots of gymnosperms and dicots. Pericamb.: Ruptures epidermis during secondary growth, seals off the vascular cambium against loss of water and other nutrients taken up from the environment; common in root and stem. Procamb.: A primary meristematic tissue, derived from the apical meristem, that gives rise to primary vascular tissues (primary phloem and xylem as well as fascicular cambium). Vascular C.: The internal part of the lateral meristem in which secondary growth occurs (secondary xylem and phloem). • Fascicular- and C.: The vascular cambium originating within a vascular, or fascicle i.e. between phloem and xylem in plants with secondary growth - alternating with the interfascicular cambium; both arise from the procambium. -

Identifying the Tree You've Selected
IDENTIFYING THE TREE YOU’VE SELECTED Blue Spruce – Pinaceae Picea Pungens Douglas Fir – Pinaceae Pseudotsuga menzieii Leaf: Evergreen, stiff, ¾ to 1 ¼ inch long, yellow-green to bluish or Leaf: Evergreen, single needles that lack woody pegs or suction white. Needles are very sharp, and have an acidic taste. cups. Needles are yellow-green to blue-green, ¾ to 1 ¼ inch long, very fragrant. Needle tips are blunt or slightly rounded. Flower: Monoecious; males yellow-brown to purple, scattered throughout trees; females purple, upright, in tops of the trees. Flower: Monoecious; males oblong, red to yellow, near branch tips; females reddish, with long bracts, occurring near branch tips. Fruit: Cones are 2 to 4 inches long, cylindrical, light brown in color. Cone scales are pointed with jagged-erose margins. Fruit: Very distinctive, 3 to 4 inches long with rounded scales. Three-lobed bracts extend beyond the cone scales and resemble Twig: Stout (when compared to other spruces), hairless, orange-brown. mouse posteriors. Maturing in August. Needles are borne on woody pegs. Bud scales are noticeably reflexed. Twig: Slender and red-brown, with long, sharp, pointed, red-brown Bark: Gray to red-brown, young trees with small, thin scales – older buds. trees developing furrows. Bark: Smooth and gray on young stems, becoming thickened, red- Form: A medium to large tree with pyramidal form. Branches appear brown with ridges and deep furrows. layered, especially with age. Form: A pyramidal crown that is somewhat open and self-prunes poorly. Stems are characteristically straight. Ponderosa Pine – Pinaceae Pinus ponderosa White Fir – Pinaceae Abies concolor Leaf: Evergreen, 5 to 10 inches long, with three (sometimes 2) tough, Leaf: Flattened needles, silvery blue-green both above and below, 2 yellow-green needles per fascicle.