Zayanah Lagoon. Benghazi- Libya *Aisha
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ABSTRACT Title of Dissertation: PATTERNS IN
ABSTRACT Title of Dissertation: PATTERNS IN DIVERSITY AND DISTRIBUTION OF BENTHIC MOLLUSCS ALONG A DEPTH GRADIENT IN THE BAHAMAS Michael Joseph Dowgiallo, Doctor of Philosophy, 2004 Dissertation directed by: Professor Marjorie L. Reaka-Kudla Department of Biology, UMCP Species richness and abundance of benthic bivalve and gastropod molluscs was determined over a depth gradient of 5 - 244 m at Lee Stocking Island, Bahamas by deploying replicate benthic collectors at five sites at 5 m, 14 m, 46 m, 153 m, and 244 m for six months beginning in December 1993. A total of 773 individual molluscs comprising at least 72 taxa were retrieved from the collectors. Analysis of the molluscan fauna that colonized the collectors showed overwhelmingly higher abundance and diversity at the 5 m, 14 m, and 46 m sites as compared to the deeper sites at 153 m and 244 m. Irradiance, temperature, and habitat heterogeneity all declined with depth, coincident with declines in the abundance and diversity of the molluscs. Herbivorous modes of feeding predominated (52%) and carnivorous modes of feeding were common (44%) over the range of depths studied at Lee Stocking Island, but mode of feeding did not change significantly over depth. One bivalve and one gastropod species showed a significant decline in body size with increasing depth. Analysis of data for 960 species of gastropod molluscs from the Western Atlantic Gastropod Database of the Academy of Natural Sciences (ANS) that have ranges including the Bahamas showed a positive correlation between body size of species of gastropods and their geographic ranges. There was also a positive correlation between depth range and the size of the geographic range. -

Portadas 21 (1)
© Sociedad Española de Malacología Iberus , 21 (1): 115-127, 2003 Four new Euthria (Mollusca, Buccinidae) from the Cape Verde archipelago, with comments on the validity of the genus Cuatro nuevas Euthria (Mollusca, Buccinidae) del archipiélago de Cabo Verde con comentarios sobre la validez del género Emilio ROLÁN *, António MONTEIRO ** and Koen FRAUSSEN *** Recibido el 2-XII-2002. Aceptado el 22-I-2003 ABSTRACT Four species, collected in the Cape Verde Islands, are described as new and assigned to the genus Euthria M. E. Gray, 1830. The new species are compared with other taxa from the Mediterranean Sea and the Cape Verde Archipelago. The genera Euthria , and Buccin - ulum are compared and the significance of the differences between them is discussed, leading to the conclusion that both genera are valid and should be kept separated. RESUMEN Se describen cuatro especies nuevas del género Euthria M. E. Gray, 1830 recogidas en aguas circalitorales del archipiélago de Cabo Verde. Las nuevas especies son compara - das con otros taxones congenéricos existentes en el Mediterráneo y en el propio archip - iélago. Recientemente, el género Euthria, únicamente conocido del Atlántico oriental, ha sido sinonimizado con Buccinulum , que posee especies en el Indo-Pacífico, por lo que se comparan ambos géneros y se discute el valor de las diferencias entre ellos, considerando finalmente que ambos son válidos, y deben mantenerse separados. KEY WORDS: Euthria , Buccinulum , Cape Verde Archipelago, new taxa. PALABRAS CLAVE : Euthria , Buccinulum , Archipiélago de Cabo Verde, nuevos táxones. INTRODUCTION During the last few years, the genus them separate, as commented upon in Euthria Gray, 1850 has frequently been “Remarks” of this paper. -

The Evolution of the Molluscan Biota of Sabaudia Lake: a Matter of Human History
SCIENTIA MARINA 77(4) December 2013, 649-662, Barcelona (Spain) ISSN: 0214-8358 doi: 10.3989/scimar.03858.05M The evolution of the molluscan biota of Sabaudia Lake: a matter of human history ARMANDO MACALI 1, ANXO CONDE 2,3, CARLO SMRIGLIO 1, PAOLO MARIOTTINI 1 and FABIO CROCETTA 4 1 Dipartimento di Biologia, Università Roma Tre, Viale Marconi 446, I-00146 Roma, Italy. 2 IBB-Institute for Biotechnology and Bioengineering, Center for Biological and Chemical Engineering, Instituto Superior Técnico (IST), 1049-001, Lisbon, Portugal. 3 Departamento de Ecoloxía e Bioloxía Animal, Universidade de Vigo, Lagoas-Marcosende, Vigo E-36310, Spain. 4 Stazione Zoologica Anton Dohrn, Villa Comunale, I-80121 Napoli, Italy. E-mail: [email protected] SUMMARY: The evolution of the molluscan biota in Sabaudia Lake (Italy, central Tyrrhenian Sea) in the last century is hereby traced on the basis of bibliography, museum type materials, and field samplings carried out from April 2009 to Sep- tember 2011. Biological assessments revealed clearly distinct phases, elucidating the definitive shift of this human-induced coastal lake from a freshwater to a marine-influenced lagoon ecosystem. Records of marine subfossil taxa suggest that previous accommodations to these environmental features have already occurred in the past, in agreement with historical evidence. Faunal and ecological insights are offered for its current malacofauna, and special emphasis is given to alien spe- cies. Within this framework, Mytilodonta Coen, 1936, Mytilodonta paulae Coen, 1936 and Rissoa paulae Coen in Brunelli and Cannicci, 1940 are also considered new synonyms of Mytilaster Monterosato, 1884, Mytilaster marioni (Locard, 1889) and Rissoa membranacea (J. -

Marine Shells of the Western Coast of Flordia
wm :iii! mm ilili ! Sfixing cHdL J^oad .Sandivicl'i, j\{ai.i.ach.u±£.tti. icuxucm \^*^£ FRONTISPIECE Photo by Ruth Bernhard Spondylus americanus Hermann MARINE SHELLS f>4 OF THE WESTERN COAST OF FLORIDA By LOUISE M. PERRY AND JEANNE S. SCHWENGEL With Revisions and Additions to Louise M. Perry's Marine Shells of the Southwest Coast of Florida Illustrations by W. Hammersley Southwick, Axel A. Olsson, and Frank White March, 1955 PALEONTOLOGICAL RESEARCH INSTITUTION ITHACA, NEW YORK U. S. A. MARINE SHELLS OF THE SOUTHWEST COAST OF FLORIDA printed as Bulletins of American Paleontology, vol. 26, No. 95 First printing, 1940 Second printing, 1942 Copyright, 1955, by Paleontological Research Institution Library of Congress Catalog Card Number: 5-^-12005 Printed in the United States of America // is perhaps a more fortunate destiny to have a taste for collecting shells than to be born a millionaire. Robert Louis Stevenson imeters 50 lllllllllllllllllllllllllllll II II III nil 2 Inches CONTENTS Page Preface by reviser 7 Foreword by Wm. J. Clench 9 Introduction 11 Generalia 13 Collection and preparation of specimens 17 Systematic descriptions 24 Class Amphineura :. 24 Class Pelecypoda 27 Class Scaphopoda 97 Class Gasteropoda 101 Plates 199 Index 311 PREFACE BY THE REVISER It has been a privilege to revise Louise M. Perry's fine book on "Marine Shells of Southwest Florida", to include her studies on eggs and larvae of mollusks; and to add descriptions and illustra- tions of several newly discovered shells thus making it a more com- prehensive study of the molluscan life of western Florida. The work that I have done is only a small return to Dr. -

Mollusc Fauna of Iskenderun Bay with a Checklist of the Region
www.trjfas.org ISSN 1303-2712 Turkish Journal of Fisheries and Aquatic Sciences 12: 171-184 (2012) DOI: 10.4194/1303-2712-v12_1_20 SHORT PAPER Mollusc Fauna of Iskenderun Bay with a Checklist of the Region Banu Bitlis Bakır1, Bilal Öztürk1*, Alper Doğan1, Mesut Önen1 1 Ege University, Faculty of Fisheries, Department of Hydrobiology Bornova, Izmir. * Corresponding Author: Tel.: +90. 232 3115215; Fax: +90. 232 3883685 Received 27 June 2011 E-mail: [email protected] Accepted 13 December 2011 Abstract This study was performed to determine the molluscs distributed in Iskenderun Bay (Levantine Sea). For this purpose, the material collected from the area between the years 2005 and 2009, within the framework of different projects, was investigated. The investigation of the material taken from various biotopes ranging at depths between 0 and 100 m resulted in identification of 286 mollusc species and 27542 specimens belonging to them. Among the encountered species, Vitreolina cf. perminima (Jeffreys, 1883) is new record for the Turkish molluscan fauna and 18 species are being new records for the Turkish Levantine coast. A checklist of Iskenderun mollusc fauna is given based on the present study and the studies carried out beforehand, and a total of 424 moluscan species are known to be distributed in Iskenderun Bay. Keywords: Levantine Sea, Iskenderun Bay, Turkish coast, Mollusca, Checklist İskenderun Körfezi’nin Mollusca Faunası ve Bölgenin Tür Listesi Özet Bu çalışma İskenderun Körfezi (Levanten Denizi)’nde dağılım gösteren Mollusca türlerini tespit etmek için gerçekleştirilmiştir. Bu amaçla, 2005 ve 2009 yılları arasında sürdürülen değişik proje çalışmaları kapsamında bölgeden elde edilen materyal incelenmiştir. -

The Upper Miocene Gastropods of Northwestern France, 4. Neogastropoda
Cainozoic Research, 19(2), pp. 135-215, December 2019 135 The upper Miocene gastropods of northwestern France, 4. Neogastropoda Bernard M. Landau1,4, Luc Ceulemans2 & Frank Van Dingenen3 1 Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, The Netherlands; Instituto Dom Luiz da Universidade de Lisboa, Campo Grande, 1749-016 Lisboa, Portugal; and International Health Centres, Av. Infante de Henrique 7, Areias São João, P-8200 Albufeira, Portugal; email: [email protected] 2 Avenue Général Naessens de Loncin 1, B-1330 Rixensart, Belgium; email: [email protected] 3 Cambeenboslaan A 11, B-2960 Brecht, Belgium; email: [email protected] 4 Corresponding author Received: 2 May 2019, revised version accepted 28 September 2019 In this paper we review the Neogastropoda of the Tortonian upper Miocene (Assemblage I of Van Dingenen et al., 2015) of northwestern France. Sixty-seven species are recorded, of which 18 are new: Gibberula ligeriana nov. sp., Euthria presselierensis nov. sp., Mitrella clava nov. sp., Mitrella ligeriana nov. sp., Mitrella miopicta nov. sp., Mitrella pseudoinedita nov. sp., Mitrella pseudoblonga nov. sp., Mitrella pseudoturgidula nov. sp., Sulcomitrella sceauxensis nov. sp., Tritia turtaudierei nov. sp., Engina brunettii nov. sp., Pisania redoniensis nov. sp., Pusia (Ebenomitra) brebioni nov. sp., Pusia (Ebenomitra) pseudoplicatula nov. sp., Pusia (Ebenomitra) renauleauensis nov. sp., Pusia (Ebenomitra) sublaevis nov. sp., Episcomitra s.l. silvae nov. sp., Pseudonebularia sceauxensis nov. sp. Fusus strigosus Millet, 1865 is a junior homonym of F. strigosus Lamarck, 1822, and is renamed Polygona substrigosa nom. nov. Nassa (Amycla) lambertiei Peyrot, 1925, is considered a new subjective junior synonym of Tritia pyrenaica (Fontannes, 1879). -

Florida Keys Species List
FKNMS Species List A B C D E F G H I J K L M N O P Q R S T 1 Marine and Terrestrial Species of the Florida Keys 2 Phylum Subphylum Class Subclass Order Suborder Infraorder Superfamily Family Scientific Name Common Name Notes 3 1 Porifera (Sponges) Demospongia Dictyoceratida Spongiidae Euryspongia rosea species from G.P. Schmahl, BNP survey 4 2 Fasciospongia cerebriformis species from G.P. Schmahl, BNP survey 5 3 Hippospongia gossypina Velvet sponge 6 4 Hippospongia lachne Sheepswool sponge 7 5 Oligoceras violacea Tortugas survey, Wheaton list 8 6 Spongia barbara Yellow sponge 9 7 Spongia graminea Glove sponge 10 8 Spongia obscura Grass sponge 11 9 Spongia sterea Wire sponge 12 10 Irciniidae Ircinia campana Vase sponge 13 11 Ircinia felix Stinker sponge 14 12 Ircinia cf. Ramosa species from G.P. Schmahl, BNP survey 15 13 Ircinia strobilina Black-ball sponge 16 14 Smenospongia aurea species from G.P. Schmahl, BNP survey, Tortugas survey, Wheaton list 17 15 Thorecta horridus recorded from Keys by Wiedenmayer 18 16 Dendroceratida Dysideidae Dysidea etheria species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 19 17 Dysidea fragilis species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 20 18 Dysidea janiae species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 21 19 Dysidea variabilis species from G.P. Schmahl, BNP survey 22 20 Verongida Druinellidae Pseudoceratina crassa Branching tube sponge 23 21 Aplysinidae Aplysina archeri species from G.P. Schmahl, BNP survey 24 22 Aplysina cauliformis Row pore rope sponge 25 23 Aplysina fistularis Yellow tube sponge 26 24 Aplysina lacunosa 27 25 Verongula rigida Pitted sponge 28 26 Darwinellidae Aplysilla sulfurea species from G.P. -

Caenogastropoda: Hydrobiidae) in the Caucasus
Folia Malacol. 25(4): 237–247 https://doi.org/10.12657/folmal.025.025 AGRAFIA SZAROWSKA ET FALNIOWSKI, 2011 (CAENOGASTROPODA: HYDROBIIDAE) IN THE CAUCASUS JOZEF GREGO1, SEBASTIAN HOFMAN2, LEVAN MUMLADZE3, ANDRZEJ FALNIOWSKI4* 1Horná Mičiná 219, 97401 Banská Bystrica, Slovakia 2Department of Comparative Anatomy, Institute of Zoology, Jagiellonian University, Cracow, Poland 3Institute of Zoology Ilia State University, Tbilisi, Georgia 4Department of Malacology, Institute of Zoology, Jagiellonian University, Cracow, Poland (e-mail: [email protected]) *corresponding author ABSTRACT: Freshwater gastropods of the Caucasus are poorly known. A few minute Belgrandiella-like gastropods were found in three springs in Georgia. Molecular markers: mitochondrial cytochrome oxidase subunit I (COI) and nuclear histone (H3) were used to infer their phylogenetic relationships. The phylogenetic trees placed them most closely to Agrafia from continental Greece. The p-distances indicated that two species occurred in the three localities. Two specimens from Andros Island (Greece) were also assigned to the genus Agrafia. The p-distances between the four taxa, most probably each representing a distinct species, were within the range of 0.026–0.043 for H3, and 0.089–0.118 for COI. KEY WORDS: spring snail, DNA, Georgia, Andros, phylogeny INTRODUCTION The freshwater gastropods of Georgia, as well as Graziana Radoman, 1975, Pontobelgrandiella Radoman, those of all the Caucasus, are still poorly studied; this 1973, and Alzoniella Giusti et Bodon, 1984. They are concerns especially the minute representatives of the morphologically distinguishable but only slightly Truncatelloidea. About ten species inhabiting karst different, and molecularly represent not closely re- springs and caves have been found so far (SHADIN lated lineages. -

A Survey of the Littoral Gastropoda of the Netherlands Antilles And
STUDIES ON THE FAUNA OF CURAÇAO AND OTHER CARIBBEAN ISLANDS: No. 31 A Survey of the Littoral Gastropoda of the Netherlands Antilles and other Caribbean Islands by H.E. Coomans (Zoologisch Museum, Amsterdam) This brief is based the material Dr. survey on collected by P. WagenaarHummelinck in 1936/37, 1948/49, and 1955. Station numbers only are cited; they refer to the “Description of new localities” in Volume IV of this series (1953; marine habitats p. 56-77) and to a “Third list oflocalities”which willbe published in a forthcoming volume. Other localities, which are not numbered, described in the are, as a rule, briefly text. Material assembled by a few other collectors has been added to Hummelinck’s collection. The names of the collectors are always mentioned, abbreviated as follows: Av.: R. Aveledo, Caracas Ga.: Wiesje and Hendrikje, Be.: J. G. van den Bergh, Aruba the two little daughters of BL. : T. Blok, Curaçao Mr. and Mrs. K. J. van Bo.: Mrs van den Bos, St Maarten Gaalen, Aruba. Co.: R. M. Collens, Tobago Za. : J. S. Zaneveld, Curaçao. For the sake of completeness the material discussed by TERA VAN BENTHEM in her "Marine Molluscs of the JUTTING paper on Island of Curasao" (1927) has been included (indicated by V.B.J.), few together with a other data on Netherlands Antilles Gastropoda from the literature of the subject, including those mentioned by J. J. SCHEPMAN in his contribution (1915) to the Encyclopaedie van Nederlandsch West-Indie (indicated by SCH.). 43 of his Dr. HUMMELINCK informed me that a considerable part marine material has not yet been examined for the presence of Gastropoda, and that the majority of the samples collected by Dr. -
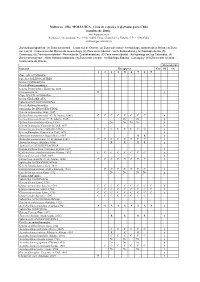
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

Gastropoda: Truncatellidae) from Florida, USA
THE NAUTILUS 130(1):13–16, 2016 Page 13 Three new species of Neogene Truncatella Risso, 1840 (Gastropoda: Truncatellidae) from Florida, USA Gary W. Schmelz Roger W. Portell 5575 Dogwood Way Florida Museum of Natural History Naples, FL 34116 University of Florida [email protected] 1659 Museum Road Gainesville, FL 32611 [email protected] ABSTRACT were discovered indicate that they were shallow water, marine environments. Three new species of truncatellid gastropods are described and illustrated. Two species are from the lower Miocene Chipola Formation of Calhoun County, Florida, and one is from upper Pliocene to lower Pleistocene deposits of the upper? Pinecrest MATERIALS AND METHODS beds, Tamiami Formation of Sarasota County, Florida. All three new species are believed to have inhabited shallow water, Specimens of Truncatella species from the lower marine environments. Miocene Chipola Formation were collected from two localities in the Florida Museum of Natural History, Invertebrate Paleontology Division (FLMNH IP) Direc- tory along the Chipola River in Calhoun County, Florida (Figure 1). One specimen was removed from matrix at FLMNH IP CA004, and two were collected from sub- INTRODUCTION merged deposits at FLMNH IP CA018. All sampled sediments were in situ. Furthermore, from the latter site, Gastropods of the family Truncatellidae display the ability pieces of loosely consolidated fossiliferous sandstone were to traverse vast distances of ocean to populate tropical and removed from underwater ledges with the aid of SCUBA. warm temperate environments. This ability to inhabit After removal, the material was brought to the FLMNH distant shorelines is accomplished by attaching them- IP laboratory for serial screen washing, air dried, and then selves or egg capsules to various pieces of flotsam and sediments were picked under stereomicroscope. -

Of the So-Called Cape Cominella, Elonyata, Dunk., and Tigrina, Kien. It Has Further the Characteristic That the Interior Cusp Of
COOKE: OX GEXEHIC rosmox OF XOHTIIIA. 235 of the so-called Cape Cominella, elonyata, Dunk., and tigrina, Kien. It has further the characteristic that the interior cusp of the lateral is serrated all along the inner edge. Group Ii.—Khachidian tooth tricuspid on a rather narrow base, base strongly arched below, and more or less prolonged into wings; laterals bicuspid, simple, outer cusp the longer and narrower. To this type belong the Magellanic species antarctica, Smith, fuscata, I3rng., fuscata, I3rug., var. curia, Prest., and innocens, Smith. There is strong reason to suspect that Preston's var. curia of fuscata, Brug.,1 is a distinct species from fuscata; the three rhachidian cusps are differently shaped, and set at a different angle.2 It appears from von Martens' description3 of the radula of his _£". chlorotica that that species also belongs to this group, and here too must be classified the West American II. dira, Eeove, which possesses a tricuspid rhachidian, set on a base which is rounded above, and deeply arched below, sides produced into wings ; laterals bicuspid,4 simple. A comparison of the radulao of Group 4 with those of the Comindlm of New Zealand [anlea, p. 228) will suggest the conclusion that the Magellanic Euthrias are in fact Cominellas, and should be classified as such. It is noticeable that, in the Talklands at least, "Euthria" occurs on muddy shores, and Mr. Iredalo tells me that in New Zealand and on Norfolk Island Cominella is always found associated with mud. EXPLANATION OF FIGURES. 1. Euthria cornea, L.: Naples. 2. ,, linca, Mart.: New Zealand.