HSOA Journal of Environmental Science: Current Research Research Article Special Issue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Persistent High Fertility in Cameroon: Young People Recount Obstacles and Enabling Factors to Use of Contraceptives
Persistent high fertility in Cameroon: young people recount obstacles and enabling factors to use of Contraceptives Authors: Maurice Kube 1,2,3*, Abeng Charles 2, Ndop Richard 2, Ankiah George 3 Institutions: 1 Department of Nursing, College of Health Sciences University of Buea, Cameroon; 2 Department of Obstetrics and Gynecology, FMBS University of Yaounde I, Yaounde, Cameroon; 3 Department of Public Health Sciences, UBA, Bamenda, Cameroon. Background Half of the world’s population is in or entering their child bearing years. Consequently there is tremendous need for contraceptive use, especially in areas with high fertility [1]. This is particularly true in Cameroon where the persistent high fertility (6.7 children per woman) is contributing to the high maternal morbidity and mortality (435/100,000 live births) as well as the rapidly growing population (3.2%) [2-4]. By comparison, a woman in two neighboring countries Gabon and Tchad will have an average of 4.5 and 2.8 children in her lifetime respectively [5]. Maternal mortality is further increased by unintended pregnancies resulting in unsafely induced abortions [4]. High fertility and high maternal morbidity and mortality not only strain individuals, families, and public resources, but also hinder opportunities for economic development [6]. Use of contraceptives has the potential to avert unplanned births, decrease maternal morbidity and mortality, increase welfare and protect future generations [6, 7]. In 2009, 49 percent of the Cameroonian population was below 15 years and 20 percent was between the age of 15 and 24[5]. A large number of young people in Cameroon are thus in or soon reaching their reproductive age and thus have a potential risk of unplanned and unwanted pregnancy [2]. -
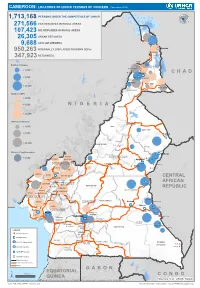
Page 1 C H a D N I G E R N I G E R I a G a B O N CENTRAL AFRICAN
CAMEROON: LOCATIONS OF UNHCR PERSONS OF CONCERN (November 2019) 1,713,168 PERSONS UNDER THE COMPETENCENIGER OF UNHCR 271,566 CAR REFUGEES IN RURAL AREAS 107,423 NIG REFUGEES IN RURAL AREAS 26,305 URBAN REFUGEES 9,688 ASYLUM SEEKERS 950,263 INTERNALLY DISPLACED PERSONS (IDPs) Kousseri LOGONE 347,923 RETURNEES ET CHARI Waza Limani Magdeme Number of refugees EXTRÊME-NORD MAYO SAVA < 3,000 Mora Mokolo Maroua CHAD > 5,000 Minawao DIAMARÉ MAYO TSANAGA MAYO KANI > 20,000 MAYO DANAY MAYO LOUTI Number of IDPs < 2,000 > 5,000 NIGERIA BÉNOUÉ > 20,000 Number of returnees NORD < 2,000 FARO MAYO REY > 5,000 Touboro > 20,000 FARO ET DÉO Beke chantier Ndip Beka VINA Number of asylum seekers Djohong DONGA < 5,000 ADAMAOUA Borgop MENCHUM MANTUNG Meiganga Ngam NORD-OUEST MAYO BANYO DJEREM Alhamdou MBÉRÉ BOYO Gbatoua BUI Kounde MEZAM MANYU MOMO NGO KETUNJIA CENTRAL Bamenda NOUN BAMBOUTOS AFRICAN LEBIALEM OUEST Gado Badzere MIFI MBAM ET KIM MENOUA KOUNG KHI REPUBLIC LOM ET DJEREM KOUPÉ HAUTS PLATEAUX NDIAN MANENGOUBA HAUT NKAM SUD-OUEST NDÉ Timangolo MOUNGO MBAM ET HAUTE SANAGA MEME Bertoua Mbombe Pana INOUBOU CENTRE Batouri NKAM Sandji Mbile Buéa LITTORAL KADEY Douala LEKIÉ MEFOU ET Lolo FAKO AFAMBA YAOUNDE Mbombate Yola SANAGA WOURI NYONG ET MARITIME MFOUMOU MFOUNDI NYONG EST Ngarissingo ET KÉLLÉ MEFOU ET HAUT NYONG AKONO Mboy LEGEND Refugee location NYONG ET SO’O Refugee Camp OCÉAN MVILA UNHCR Representation DJA ET LOBO BOUMBA Bela SUD ET NGOKO Libongo UNHCR Sub-Office VALLÉE DU NTEM UNHCR Field Office UNHCR Field Unit Region boundary Departement boundary Roads GABON EQUATORIAL 100 Km CONGO ± GUINEA The boundaries and names shown and the designations used on this map do not imply official endorsement or acceptance by the United Nations Sources: Esri, USGS, NOAA Source: IOM, OCHA, UNHCR – Novembre 2019 Pour plus d’information, veuillez contacter Jean Luc KRAMO ([email protected]). -

The$Use$Of$New$Information$And
Advances)in)Social)Sciences)Research)Journal)–)Vol.3,)No.1) ! Publication)Date:!Jan.!25,!2016! DoI:10.14738/assrj.31.1310.! Lengha,'T.'N.'(2016).'The'Use'of'New'Information'and'Communication'Technologies'as'an'Education'Tool'in'the'Fight'Against' ! HIV/AIDS'in'Fundong,'Northwest'Region,'Cameroon.)Advances)in)Social)Sciences)Research)Journal,)3(1))51B60.' ! The$Use$of$New$Information$and$Communication*Technologies* as#an#Education#Tool#in#the#Fight#Against#HIV/AIDS%in#Fundong," Norhtwest)Region,"Cameroon) ! Tohnain)Nobert)Lengha) Department!of!Agricultural!Extension!and!Rural!Sociology,! Faculty!of!Agronomy!and!Agricultural!sciences,! University!of!Dschang,!Cameroon! ! Abstract) Fundong,) a) rural) town) found) in) the) Northwest) Region) of) Cameroon) is) located) on) latitude)10°)14’W)and)11°15’)E,)between)longitudes)6°)27’)and)8°)26’N.)))The)town)enjoys) the)privilege)of)being,)not)just)the)headquarter)of)Boyo)Division,)but)also)of)Fundong) Central) SubQDivision.) The) incidence) of) HIV/AIDS) is) critical) in) the) area) as) there) are) several)practices)like)the)scarification)of)the)body)to)apply)concoctions)common)in)the) area)which,)may)help)predispose)the)population)to)HIV/AIDS)infection.)The)affluence) that) characterise) this) small) rural) town) favours) highQrisk) behaviours,) which) expose) individuals)concerned)to)HIV/AIDS.)In)order)to)address)the)main)objective)of)the)study,) which) is) the) use) of) information) and) communication) technologies) in) the) fight) against) HIV/AIDS,) data) were) collected) at) ) the) group) -

Shelter Cluster Dashboard NWSW052021
Shelter Cluster NW/SW Cameroon Key Figures Individuals Partners Subdivisions Cameroon 03 23,143 assisted 05 Individual Reached Trend Nigeria Furu Awa Ako Misaje Fungom DONGA MANTUNG MENCHUM Nkambe Bum NORD-OUEST Menchum Nwa Valley Wum Ndu Fundong Noni 11% BOYO Nkum Bafut Njinikom Oku Kumbo Belo BUI Mbven of yearly Target Njikwa Akwaya Jakiri MEZAM Babessi Tubah Reached MOMO Mbeggwi Ngie Bamenda 2 Bamenda 3 Ndop Widikum Bamenda 1 Menka NGO KETUNJIA Bali Balikumbat MANYU Santa Batibo Wabane Eyumodjock Upper Bayang LEBIALEM Mamfé Alou OUEST Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Fontem Nguti KOUPÉ HNO/HRP 2021 (NW/SW Regions) Toko MANENGOUBA Bangem Mundemba SUD-OUEST NDIAN Konye Tombel 1,351,318 Isangele Dikome value Kumba 2 Ekondo Titi Kombo Kombo PEOPLE OF CONCERN Abedimo Etindi MEME Number of PoC Reached per Subdivision Idabato Kumba 1 Bamuso 1 - 100 Kumba 3 101 - 2,000 LITTORAL 2,001 - 13,000 785,091 Mbongé Muyuka PEOPLE IN NEED West Coast Buéa FAKO Tiko Limbé 2 Limbé 1 221,642 Limbé 3 [ Kilometers PEOPLE TARGETED 0 15 30 *Note : Sources: HNO 2021 PiN includes IDP, Returnees and Host Communi�es The boundaries and names shown and the designations used on this map do not imply official endorsement or acceptance by the United Nations Key Achievement Indicators PoC Reached - AGD Breakdouwn 296 # of Households assisted with Children 27% 26% emergency shelter 1,480 Adults 21% 22% # of households assisted with core 3,769 Elderly 2% 2% relief items including prevention of COVID-19 21,618 female male 41 # of households assisted with cash for rental subsidies 41 Households Reached Individuals Reached Cartegories of beneficiaries reported People Reached by region Distribution of Shelter NFI kits integrated with COVID 19 KITS in Matoh town. -

From Migrants to Nationals, from Nationals To
International Journal of Latest Engineering and Management Research (IJLEMR) ISSN: 2455-4847 www.ijlemr.com || Volume 03 - Issue 06 || June 2018 || PP. 10-19 From Nomads to Nationals, From Nationals to Undesirable Elements: The Case of the Mbororo/Fulani in North West Cameroon 1916-2008 A Historical Investigation Jabiru Muhammadou Amadou (Phd) Higher Teacher Training College Department of History The University of Yaounde 1 Abstract: The Fulani (Mbororo) are a minority group perceived as migrants and strangers by local North West groups who consider themselves their hosts and landlords. They are predominantly nomadic people located almost exclusively within the savannah zone of West and Central Africa. Their original homeland is said to be the Senegambia region. From Senegal, the Fulani continued their movement along side their cattle and headed to Northern Nigeria. Uthman Dan Fodio‟s 19th century jihad movement and epidemic outbreaks force them to move from Northern Nigeria to Northern Cameroon. From Northern Cameroon they moved down South and started penetrating the North West Region in the early 20th century. This article critically examines and considers the case of the Fulani in the North West Region of Cameroon and their recent claims to regional citizenship and minority status. The paper begins by presenting the migration, settlement and ultimate acquisition of the status of nationals by the nomadic cattle Fulani in North West Cameroon. It also analysis the difficulties encountered by this nomadic group to fully integrate themselves into the region. In the early 20th century (more precisely 1916) when they arrived the North West Region, they were warmly received by their hosts. -

Cameroon:NW/SW Highlights Needs 690K 414K 63K1 52 $9.5M
Cameroon:NW/SW WASH Update April 2020 Hand washing sensitization of community members in the North West region. Photo by NRC Highlights Needs In order to contain the spread of COVID-19, WASH partners have scaled up community 690k People in need of WASH engagement activities. More than 116,000 services in NW/SW people were reached through COVID 19 sensitization sessions in April. 414k In response to the COVID 19 pandemic, Targeted ReachOut, with support from UNICEF, 1 installed 250 communal hand washing 63k IDPs & Returnees stations in Ekondo Titi. More than 12,500 people are expected to benefit. 52 More than 10,000 individuals received WASH partners WASH and hygiene kits from WASH partners in April. $9.5m In April, about 1,600 people benefitted from required for WASH improved water supply as a result of US$9.5M installation of water distribution systems by WASH partners. Reguired WASH partners provided improved sanitation facilities to 400 people. US$0.2M Funded 1 IDP Tracking Database, May 2020 (Note: This figure is the latest displacement figure as of 16 May 2020) Website: https://www.humanitarianresponse.info/en/operations/cameroon/water-sanitation-hygiene For more information contact Wash Cluster Coordinator: Nchunguye Festo Vyagusa Email: [email protected] WATER Plan International, in collaboration with UNICEF completed rehabilitation of a water distribution system in Fundong, Boyo division, reaching 1,650 individuals with safe drinking water. Rehabilitation of water systems in Bamenda 2 subdivision in Mezam is ongoing. Plan International, supported by UNICEF is planning to rehabilitate two water distribution systems in Babessi sub-division of Ngo- Ketunjia division in May. -

CAMEROON Bulletin # 10
CAMEROON Bulletin # 10 CAMEROON North-West and South-West, Health Cluster Update, 08-15 July 2020 16 July, 2020 COVID-19 SITUATION HIGHLIGHTS (as of 16 July 2020, 6:00 PM) CFR 4 % GLOBAL: 13,589,275+ 584,990+ Confirmed Cases To t a l Deaths 2% AFRICAN 644,333+ 14,047+ REGION: Confirmed Cases To t a l Deaths 2% CAMEROON: 16, 157+ 373 Confirmed Cases To t a l Deaths SOUTH - WEST REGION (as of 16 July 2020): 6% 624 2,548+ 36 820,000+ Confirmed Cases Total Tested To t a l Deaths Total Passengers Screened NORTH - WEST REGION (as of 14 July 2020): 10% WHO SW Region finalizing the distribution list of received COVID-19 Medical supplies to key health facilities from various donors Photo: WHO/ Venkat Dheeravath 610 2,600+ 61 856,000 + IMMEDIATE NEEDS: Confirmed Cases Total Tested To t a l Deaths Total Passengers Screened COVID-19 CASES PER SEX IN NWSW REGION: • More medical supplies (oxygen concentrator/cylinders, COVID-19 treatment kits) in both regions. 60% 40% Source: WHO; Ministry of Public Male Female Health-Cameroon, Johns Hopkins; nCoV • More health districts need to be trained and provided with tools for effective KEY HIGHLIGHTS: contact tracing. • In NW region, 13 out of the I9 health districts reported confirmed cases of COVID-l9. 72 % of the cases have been recorded in Bamenda and 12% • Stronger sensitization is required to counter the misinformation about the of total cases recorded in Fundong health district. management of COVID-19 in the communities and at health facilities. -

Masculinity and Female Resistance in the Rice Economy in Meteh/Menchum Valley Bu, North West Cameroon, 1953 – 2005
Journal of Sustainable Development in Africa (Volume 15, No.7, 2013) ISSN: 1520-5509 Clarion University of Pennsylvania, Clarion, Pennsylvania MASCULINITY AND FEMALE RESISTANCE IN THE RICE ECONOMY IN METEH/MENCHUM VALLEY BU, NORTH WEST CAMEROON, 1953 – 2005 Henry Kam Kah University of Buea, Cameroon ABSTRACT Male chauvinism and female reaction in the rice economy in Bu, Menchum Division of North West Cameroon is the subject of this investigation. The greater focus of this paper is how and why this phobia has lessened over the years in favour of female dominion over the rice economy. The point d’appui of the masculine management of the economy and the accentuating forces which have militated against their continuous domination of women in the rice sector have been probed into. Incongruous with the situation hitherto, women have farms of their own bought with their own money accumulated from other economic activities. In addition, they now employ the services of men to execute some defined tasks in the rice economy. From the copious data consulted on the rice economy and related economic endeavours, it is a truism that be it collectively and/or individually, men and women in Bu are responding willingly or not, to the changing power relations between them in the rice economy with implications for sustainable development. Keywords: Masculinity, Female Resistance, Rice Economy, Cameroon, Sustainability 115 INTRODUCTION: RELEVANCE OF STUDY AND CONCEPT OF MASCULINITY Rice is a staple food crop in Cameroon like elsewhere in Africa and other parts of the world. It has become increasingly important part of African diets especially West Africa and where local production has been insufficient due to limited access to credit (Akinbode, 2013, p. -

PC19 Inf. 12 (In English and French / En Inglés Y Francés / En Anglais Et Français)
PC19 Inf. 12 (In English and French / en inglés y francés / en anglais et français) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA CONVENCIÓN SOBRE EL COMERCIO INTERNACIONAL DE ESPECIES AMENAZADAS DE FAUNA Y FLORA SILVESTRES CONVENTION SUR LE COMMERCE INTERNATIONAL DES ESPECES DE FAUNE ET DE FLORE SAUVAGES MENACEES D'EXTINCTION ____________ Nineteenth meeting of the Plants Committee – Geneva (Switzerland), 18-21 April 2011 Decimonovena reunión del Comité de Flora – Ginebra (Suiza), 18-21 de abril de 2011 Dix-neuvième session du Comité pour les plantes – Genève (Suisse), 18 – 21 avril 2011 PRELIMINARY REPORT ON SUSTAINABLE HARVESTING OF PRUNUS AFRICANA (ROSACEAE) IN THE NORTH WEST REGION OF CAMEROON The attached information document has been submitted by the CITES Secretariat1. El documento informativo adjunto ha sido presentado por la Secretaría CITES2. Le document d'information joint est soumis par le Secrétariat CITES3. 1 The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat or the United Nations Environment Programme concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. 2 Las denominaciones geográficas empleadas en este documento no implican juicio alguno por parte de la Secretaría CITES o del Programa de las Naciones Unidas para el Medio Ambiente sobre la condición jurídica de ninguno de los países, zonas o territorios citados, ni respecto de la delimitación de sus fronteras o límites. -

Non-Commercial Use Only
Journal of Public Health in Africa 2011 ; volume 2:e10 Social stigma as an epidemio- lack of self-esteem, tribal stigma and complete rejection by society. From the 480 structured Correspondence: Dr. Dickson S. Nsagha, logical determinant for leprosy questionnaires administered, there were over- Department of Public Health and Hygiene, elimination in Cameroon all positive attitudes to lepers among the study Medicine Programme, Faculty of Health Sciences, population and within the divisions (P=0.0). University of Buea, PO Box 63, Buea, Cameroon. Dickson S. Nsagha,1,2 The proportion of participants that felt sympa- Tel. +237. 77499429.E-mail: [email protected] [email protected] Anne-Cécile Z.K. Bissek,3 thetic with deformed lepers was 78.1% [95% 4 confidence interval (CI): 74.4-81.8%] from a Sarah M. Nsagha, Key words: leprosy, social stigma, attitudes, elim- Anna L. Njunda,5 total of 480. Three hundred and ninety nine ination, Cameroon. Jules C.N. Assob,6 (83.1%) respondents indicated that they could Earnest N. Tabah,7 share a meal or drink at the same table with a Acknowledgements: the authors are grateful to Elijah A. Bamgboye,2 deformed leper (95% CI: 79.7-86.5%). Four Mr. Nsagha BN, Mr. Nsagha IG and Late Papa hundred and three (83.9%) participants indi- James Nsagha, who sponsored this study. The Alain Bankole O.O. Oyediran,2 cated that they could have a handshake and authors also thank Mr. Agyngi CT & Mr. Ideng DA Peter F. Nde,1 embrace a deformed leper (95% CI: 80.7- of the Benakuma Health Center; Mr. -

CAMEROON Bulletin # 09
CAMEROON Bulletin # 09 CAMEROON North-West and South-West, Health Cluster Update, 01-08 July 2020 09 July, 2020 COVID-19 SITUATION HIGHLIGHTS (as of 09 July 2020, 6:00 PM) CFR 5 % GLOBAL: 12,128,400+ 551,522+ Confirmed Cases To t a l Deaths 3% AFRICAN 523,782+ 12,245+ REGION: Confirmed Cases To t a l Deaths 3% CAMEROON: 15, 173+ 359 Confirmed Cases To t a l Deaths SOUTH - WEST REGION (as of 08 July 2020): 6% 580 2,015 34 740,000+ Confirmed Cases Total Tested To t a l Deaths Total Passengers Screened NORTH - WEST REGION (as of 08 July 2020): 9% Weekly COVID-19 Incident Management System Meeting at South West Regional Delegation with the Health Cluster Partners on 06 July 2020 Photo: WHO/ Venkat Dheeravath 559 2,215 55 756,000 + IMMEDIATE NEEDS: Confirmed Cases Total Tested To t a l Deaths Total Passengers Screened COVID-19 CASES PER SEX IN NWSW REGION: • More medical supplies (oxygen concentrator/cylinders, COVID-19 treatment kits) in both regions. 59% 41% Source: WHO; Ministry of Public Male Female Health-Cameroon, Johns Hopkins; nCoV • Both regions are still in need of more Personal Protective Equipment (PPE) and KEY HIGHLIGHTS: training on Infection Prevention and Control (IPC). • Early Warning Alert and Response System (EWARS) reported 15 • More health districts need to be trained and provided with tools for effective suspected COVID-19 cases and two deaths in Akwaya health district, SW contact tracing. region. • Stronger sensitization is required to counter the misinformation about the • In NW region, 13 out of the I9 health districts reported confirmed cases management of COVID-19 in the communities and at health facilities. -

The Anglophone Crisis in Cameroon: a Geopolitical Analysis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by European Scientific Journal (European Scientific Institute) European Scientific Journal December 2019 edition Vol.15, No.35 ISSN: 1857 – 7881 (Print) e - ISSN 1857- 7431 The Anglophone Crisis in Cameroon: A Geopolitical Analysis Ekah Robert Ekah, Department of 'Cultural Diversity, Peace and International Cooperation' at the International Relations Institute of Cameroon (IRIC) Doi:10.19044/esj.2019.v15n35p141 URL:http://dx.doi.org/10.19044/esj.2019.v15n35p141 Abstract Anglophone Cameroon is the present-day North West and South West (English Speaking) regions of Cameroon herein referred to as No-So. These regions of Cameroon have been restive since 2016 in what is popularly referred to as the Anglophone crisis. The crisis has been transformed to a separatist movement, with some Anglophones clamoring for an independent No-So, re-baptized as “Ambazonia”. The purpose of the study is to illuminate the geopolitical perspective of the conflict which has been evaded by many scholars. Most scholarly write-ups have rather focused on the causes, course, consequences and international interventions in the crisis, with little attention to the geopolitical undertones. In terms of methodology, the paper makes use of qualitative data analysis. Unlike previous research works that link the unfolding of the crisis to Anglophone marginalization, historical and cultural difference, the findings from this paper reveals that the strategic location of No-So, the presence of resources, demographic considerations and other geopolitical parameters are proving to be responsible for the heightening of the Anglophone crisis in Cameroon and in favour of the quest for an independent Ambazonia.