United States Patent Office Patented June 8, 1965 2
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Push Pull & Slipsheet Handling Manual
PUSH/PULL & SLIPSHEET HANDLING MANUAL Global Solutions in Materials Handling PUSH/PULL & SLIPSHEET HANDLING MANUAL Table of Contents ■ INTRODUCTION BACKGROUND & REVIEW What You May Not Know About Slipsheets . .4 Palletless Load Handling–Is It Your Future? . .6 Put It On A Slipsheet . .12 Push/Pulls Global Progress . .15 Slipsheets: The Pallet’s Successor? . .21 Are Packagers Ready For Slipsheets? . .24 Making A Case For Slipsheets . .28 Slipsheets Save Weight And Cost In The Air . .31 Germany Says No To North American Pallets! . .34 US Tells Importers: Pests Are Unpalletable . .36 Freight Processing Centers: Logistics’ Missing Link . .38 ■ COST COMPARISONS Slipsheets & Pallets: A Cost Comparison . .41 Why General Foods Converted To Slipsheets . .47 Cost Comparison Worksheets (Examples and Blanks) . .50 Slipsheets vs. Pallets – The Decision Making Process . .61 ■ CASE HISTORIES & INDUSTRY UTILIZATION Slipsheeting The World – Apple Computer . .67 Cascade Plays Key Role – Apple Computer . .68 Slipsheets Generate Hyper Savings – Apple Computer . .70 You Must Use Slipsheets – Home Depot . .74 Lift Truck Attachment Eliminates Pallet Use – Quaker State Oil . .79 Prior To Slipsheets – Speech By Jim Chase, President Jewell Foods, Inc. .84 Unitizing 30 Lb. Cans Saves 75% Labor – Pik'd Rite, Inc. .88 On-The-Job Application Report – Simplot . .89 On-The-Job Application Report – Northern Fruit . .94 Industries Using Push/Pulls . .95 1 Table of Contents (cont.) ■ CONVERTING TO SLIPSHEETS Slipsheeting – From Team To Reality . .101 Slipsheeting – Making It A Success For Suppliers . .103 Do’s & Don’ts Of Converting To Slipsheets . .111 Recommended Guidelines To Follow For Successful Push/Pull Test . .114 Slipsheet Specifications – Apple Computer . .115 Home Depot's Conversion Spurs Interest In Slipsheets . -

Platinum Motorized Treadmill
Platinum Motorized Treadmill Assembly & User Instructions- Please keep for future reference 228/7278 Important – Please read these instructions fully before assembly or use These Instructions contain important information which will help you get best from your equipment and ensure safe and correct assembly, use and maintenance. If you need help or have damaged or missing parts, call the Customer Helpline: 0345 600 1714 or visit www.argos-support.co.uk Issue 1 -8/8/18 Contents Safety Information 2-3 Components-Parts 4 Components-Fixings 5 Assembly Instructions 6-10 Workout Area 11 Exercising Information 12-26 Before starting 12 Muscle Chart 13 Warming up and Cooling down 14-15 Getting Started 16-17 Console Operation 18-25 Folding Mechanism and Locking System 26 Care and Maintenance 27-32 Exploded Parts Diagram 33 Parts List 34-35 5 1 、 Safety information Important – Please read fully before assembly or use This exercise equipment is built for optimum safety. However, certain precautions apply whenever you operate a piece of exercise equipment. Be sure to read the entire manual before you assemble, operate or use this equipment. and other related problems that are outside our Assembly control. • The product must be installed on a stable and level • Keep unsupervised children away from the surface. Do not place the treadmill on any surface equipment. that blocks air openings. To protect the floor or carpet • Disabled persons should not use the equipment from damage, place a mat under the treadmill. without a qualified person or doctor in attendance. • Assemble the item as close to its final position • Always wear appropriate workout clothing when (in the same room) as possible. -

20171 Minimum Design and Construction Requirements
Virginia Housing Development Authority 20171 Minimum Design and Construction Requirements Requirements for All Developments The following requirements were created to address issues related to the design, construction, maintenance, marketing, life cycle costs and aesthetic concerns for developments utilizing low income housing tax credits (LIHTC), and/or developments financed by the Virginia Housing Development Authority (VHDA). Submission requirements for VHDA loan applications are listed on the Architectural & Engineering Review sheet which can be found at the conclusion of the Minimum Design and Construction Requirements (MDCR). Submission requirements for the LIHTC program are contained in the tax credit application. Drawings, specifications and scope of work are to comply with the latest applicable issue of the Virginia Uniform Statewide Building Code (USBC)2, International Building Code (IBC)3, other applicable Virginia and national codes, requirements of localities, prevailing design and construction practices and the Minimum Design and Construction Requirements of VHDA. Installation of materials, equipment, products, and building systems are to be per the manufacturers’ requirements, specifications, and recommendations. All developments are to comply with accessibility requirements of USBC. Requirements for New Construction SITE WORK 1. Finished floor elevations of buildings are to be a minimum of 8 inches higher than the adjoining finished grade. When achieving an 8 inch height separation is not feasible, due to accessibility requirements or other conditions, provide an alternate solution acceptable to VHDA. 2. Areas around buildings are to be graded to have a minimum 5% slope away from foundation walls for a minimum distance of 10 feet, per IBC. Install yard drains, storm inlets, or drainage pipes under concrete walks to drain properly if the space between foundation walls and concrete walks is less than 10 feet. -

The Regional Municipality of Waterloo Contract T2016-118 Cambridge
The Regional Municipality of Waterloo Contract T2016-118 Cambridge Centre Terminal 381 Hespeler Road Cambridge, Ontario THE REGIONAL MUNICIPALITY OF WATERLOO BID NO. T2016-118 TABLE OF CONTENTS Page BIDDER'S CHECK LIST 1 SECTION A INSTRUCTIONS TO BIDDERS A-1 to A-15 SECTION B BID B-1 to B-13 ENVELOPE TEMPLATE SECTION C SUPPLEMENTAL GENERAL CONDITIONS C-1 to C-27 SECTION D SPECIAL PROVISIONS D-1 to D-2 SECTION E TECHNICAL SPECIFICATIONS E-1 to E-319 APPENDIX 1 DRAWING PACKAGE (To be downloaded as a separate attachment – 56 pages) APPENDIX 2 GEOTECHNICAL INFORMATION (To be downloaded as a separate attachment – 35 pages) CAMBRIDGE CENTRE TERMINAL T2016-118 BIDDER'S CHECK LIST Before submitting your bid, check the following points: 1. Has your bid been properly signed? ______ 2. Have you enclosed the bid deposit, ie. certified cheque or bid bond? ______ Please note that Bid Deposit shall be valid for at least 120 days from bid closing 3. Have you enclosed the Agreements to Bond, signed and sealed by your proposed surety? ______ 4. Have you listed all listed Subcontractors? ______ 5. Have you provided one copy of the bid form (Section B)? ______ 6. Are the documents complete? ______ NOTE: Your bid will be informal and may be disqualified if ANY of the foregoing points (if applicable) have not been complied with. MAKE SURE THAT YOU SEAL THE BID IN AN ENVELOPE. CAMBRIDGE CENTRE TERMINAL T2016-118 SECTION A INSTRUCTIONS TO BIDDERS CAMBRIDGE CENTRE TERMINAL T2016-118 INDEX PAGE SECTION A INSTRUCTIONS TO BIDDERS 1. -

Creative-Home-Styles.Pdf
Mouldings See pages 24–29... WindsorWindsor Plywood'sPlywood's HomeHome FinishingFinishing CatalogueCatalogue Natural live-edge lumber & slabs . See page 33... Flooring See pages 15–20... www.windsorplywood.com For Expert Advice Shop Locally Primed MDF mouldings and solid maple hardwood flooring. Home finishing has been our passion for over 45 years! Since 1969, we've specialized in hard-to- source interior and exterior home finishing products; especially wood products, including exotic woods and natural live-edge lumber and slabs. Our catalogue showcases some of the doors, flooring, mouldings, stair parts, hardware, tools and finishing products from our wide and constantly changing selection. You'll find even more in your local store! Windsor Plywood locations are locally owned and operated; often family affairs, passed down through the generations. We are committed to providing outstanding value and personalized, one-on-one service to all of our customers: homeowners, do-it-yourselfers, renovators, builders, designers, craftsmen and contractors. Regardless of the type or size of your project, we'll help you bring your vision to life, from start to finish. S i 9 n c e 1 9 6 Special Order Services. We shop the world, so you don't have to! We'll save you time by finding products that best fit your needs from the thousands of products available from our suppliers worldwide. We also provide free estimates and advice with no strings attached, even if you just need to compare the price on a couple of doors or need a quote on a whole finishing package. We are Doors Experts! Doors make the first impression and our in-store door shops provide everything you need for custom sizing, machining for hinges and locks, or installing decorative glass lites. -
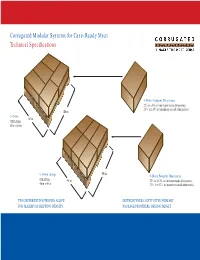
Corrugated Modular Systems for Case-Ready Meat Technical Specifications
Corrugated Modular Systems for Case-Ready Meat Technical Specifications 5-Down Footprint Dimensions 3 3 22 /4 in x 14 /4 in (minimum inside dimensions) 13 7 23 /16 in x 15 /8 in (maximum outside dimensions) 48 in 5-Down 40 in GMA Pallet 48 in x 40 in 48 in 6-Down (Long) 6-Down Footprint Dimensions GMA Pallet 3 5 40 in 22 /4 in x 12 /16 in (minimum inside dimensions) 13 3 48 in x 40 in 23 /16 in x 13 /16 in (maximum outside dimensions) TWO DIFFERENT FOOTPRINTS ALLOW DEFINED INNER CAVITY GIVES PRIMARY FOR MAXIMUM SHIPPING DENSITY PACKAGE PROVIDERS DESIGN TARGET MAKEDETAILS THE DIFFERENCE The benefits of the new corrugated modular systems for Case-Ready Meat depend upon attention to details. The systems’ specifications establish voluntary uniform transport packaging footprints, while leaving room for innovation and flexibility in package design. The Two footprintsd pallets Fits standar systems’ footprints and dimensional specifications are designed to maximize the benefits of modularity. Here are all the essential details: Maximizes payloadss Minimizes cost Inside dimensions. Container top. Lighter tare weight Inside dimensions are set as minimums The container top is not allowed to only and are intended to preclude and be more than 80 percent open, as Custom-design options prevent any portion of the transport positioned for storage and distribution. Minimum internal dimensions package (e.g., minor flaps, tabs, etc.) from incursion into the dimensionally Compression strength. specified internal dimensions. (Figure 1) The corrugated industry’s modular systems do not include minimum Outside dimensions. compression strength values (a principal Outside dimensions are set as maximums element of stacking strength), which only and are intended to preclude should be appropriately determined by any part or portion of the transport container suppliers and their customers or primary package (as shipped) from in order to perform adequately throughout extending outside the dimensionally the entire distribution system. -

TM-U590/U590P Operator’S Manual MICR Option Included
slip printer TM-U590/U590P Operator’s Manual MICR Option Included 400676902 Printer Parts and Labels EPSON Front cover Table FORWARD POWER ERROR REVERSE RELEASE SLIP RELEASE On/Off switch Slip paper Paper guide control panel * Slide the paper guide FORWARD POWER to fit the width of Document table ERROR RELEASE your paper. SLIP REVERSE RELEASE Labels 2 Ribbon installation 1 label inside Label on the document table front cover CAUTION: Caution labels for drawer kick-out and display module connectors. Quick Reference This Quick Reference will direct you to key areas of this Operator’s Manual. For a complete listing of topics, see the Contents. Printer Parts and Labels inside front cover Ordering Ribbons page viii Where to order ribbons. Setting Up the Printer page 1-1 How to set up the printer. Validating and Verifying Checks page 2-4 How to validate and verify checks using the optional Magnetic Ink Character Recognition (MICR) Reader. Solving Problems page 3-1 How to correct problems. i All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of Seiko Epson Corporation. No patent liability is assumed with respect to the use of the information contained herein. While every precaution has been taken in the preparation of this book, Seiko Epson Corporation assumes no responsibility for errors or omissions. Neither is any liability assumed for damages resulting from the use of the information contained herein. Neither Seiko Epson Corporation nor its affiliates shall be liable to the purchaser of this product or third parties for damages, losses, costs, or expenses incurred by purchaser or third parties as a result of: accident, misuse, or abuse of this product or unauthorized modifications, repairs, or alterations to this product, or (excluding the U.S.) failure to strictly comply with Seiko Epson Corporation’s operating and maintenance instructions. -

Project Submittal Package
Project Submittal Package Project: Location: Architect: General Contractor: Applicator: __ System Data Sheets __ Product Data Sheets __ Sample Warranty __ Specifications __ Details __ Web Link (click here) masterwall.com PO Box 397 • Fortson • GA • 31808 • 800-755-0825 Cemplaster Fiberstucco 09 24 00/09 25 00 Premium Fibered Stucco with a Warranty Master Wall® Cemplaster Fiberstucco is an engineered, fiber-reinforced stucco. It offers the dura- bility of traditional stucco with easier application better product consistency and durability. Cem- plaster Fiberstucco is available as a basic system (stucco & finish) or it can be upgraded with ad- ditives, rainscreen mats, continuous insulation leveling base coats and finishes. 5. Cemplaster Fiber- stuccostuccostucco from basic to highly modified 1 3 6. Leveling Base 6 Coats & Meshes Improves 4 aesthetics and perform- ance 5 7 7. Primers7. Primers Conditions the Surface 2 and improves color 1. Water Barriers from consistency simple to high tech Mas- ter Wall Rollershield air/ 8 water barrier 8. Finish Options from Superior to Elastomeric Plus 2. Continuous Insulation Option Up To reduces heat loss and meets new energy code requirements 202020 4. Metal Lath Year Labor/Material Limited from lightweight to heavy Warranty 3. Drainage Spacers from duty woven to rainscreen mats masterwall.com PO Box 397 • Fortson • GA • 31808 • 800-755-0825 Cemplaster Fiberstucco Choose your system and your warranty, or let us custom design one for you When you improve your stucco you improve your warranty. Choose the options you want for your project or give us a call and we will custom design a system for you. -

Product Reference Guide Setting the Standard for Excellence
Product Reference Guide Setting the Standard for Excellence Carlisle SynTec Systems, the flagship division of Carlisle Construction Materials (CCM), is the largest supplier of commercial roofing products in the world. Carlisle produces high-performance EPDM, TPO, PVC, and FleeceBACK® single-ply roofing membranes, a full line of polyiso and expanded polystyrene insulation, and a wide variety of solvent-based and low-VOC adhesives. With more than 55 years of manufacturing experience and billions of square feet of roofing materials sold, Carlisle continues to lead the industry by providing the best products, services, and warranty options available today. Product Reference Guide TABLE OF CONTENTS EPDM Accessories ................................................................................................................................ 1 Thermoplastic Accessories .................................................................................................................... 3 Fasteners & Plates ................................................................................................................................ 9 Adhesives, Primers, and Sealants .........................................................................................................13 Air & Vapor Barriers ..............................................................................................................................19 Insulation Reference ........................................................................................................................... -

Product List Table of Contents
09/20 . Technical statements are subject to alterations and printing errors, 12363 Systems and Components for Roof Landscapes Systems andComponentsfor Roof Product List Product Table of Contents Slip Sheet and Separation Membrane 3 Components for Green Roof Systems Root Barriers 4 Page 3 Root Barriers Accessories 5 Protection Mats 6 Drainage Elements 8 Building Protection and Drainage Elements 12 Filter Sheets 14 Drainage Channels, Balcony and Mineral Substrates / Delivery Forms 15 Terrace Channels, Terrace Grills System Substrates 16 Page 27 Substrates in Bags / Fertilizer 17 Seed Mixtures / Roof Tree Anchorage Robafix® 18 Pitched and Steep Pitched Green Roofs 19 Shear Protection Accessories 20 System Build-ups with Aquafleece® AF 300 21 Separations, Eaves Profiles, System Build-up “Roof Garden“ with Aquatec® AT 45 22 Clamping and Protection Profiles System Build-up “Stormwater Management Roof“ 23 Page 30 Inspection Chambers 24 Dam-up Irrigation 26 Drainage Channels 27 Balcony and Terrace Channel 28 Terrace Grills 29 Fall Protection and Guardrail Solutions Separations 30 Page 34 Eaves Profiles 30 Gravel Retainers 32 Clamping and Protection Profiles 33 Fall Protection Fallnet® 34 Guardrail Solutions 36 Vertically Adjustable Pedestals Elefeet® 38 Vertically Adjustable Pedestals Elefeet® Support Rail System 39 Page 38 Elefeet® Support Rail Accessories 39 Pedestals Accessories 41 Slab Pavements Accessories 42 Photovoltaics and Green Roofs 43 PV Subconstruction Accessories 45 Why have a Green Roof? 46 Photovoltaics and Green Roofs Types of Green Roofs 47 Page 43 European Technical Assessment 48 The Roofs of the Future are Green 49 Please note that this English Version of the Product List is for your information only, but is composed of 1. -

Comcolor GD Series User's Guide About This Guide
User's Guide This guide describes total operation features and specifications. Series 061-360UG-EN3 Options and functions vary depending on the model. ComColor GD9630/GD9630R ComColor GD9631/GD9631R ComColor GD7330/GD7330R Feed Tray (1,2 and 3) ✓ N/A* Connectivity of the Wrapping ✓ N/A Envelope Finisher (optional) ✓: Available N/A: Not available * The [Mixed Size Original], [Add Cover], [Slip Sheet], and [Paper Tray Relay] functions in Copy mode, Print mode, and Storage mode are not available. The [Multi-part Paper Printing] function of the printer driver is not available. The [Mixed Size Original] function in Scan mode is available only for reading. This device is compliant with the substance emission inspections of the German environmental label "RAL-UZ171". CAUTION After reading this guide, keep it handy for future reference. The "Safety Precautions" are provided in the supplemental Safety Information. Be sure to read through them prior to use. ComColor GD Series User's Guide About This Guide Preface Thank you for your purchase of a ComColor GD Series product. This ComColor GD Series product is a network-compatible inkjet printer that performs high-speed color printing. This machine uses five colors of ink, cyan, magenta, yellow, black, and gray, to enable smooth expression of gradations while improving the reproduction of highlights and density of black in color printing. Besides output of data and management of the printer from a computer, this machine can be used in combination with an optional scanner for various functions such as copying originals and saving or utilizing scanned data. The five guides described below are supplied with this machine. -

BROCHURE ABRIDGED Oeattachments.Com | 833-OEA-KAUP 632-5287 OEAK-BROC-2021-04-29 ABOUT US
North American Specifications German Engineering 1 BROCHURE ABRIDGED OEattachments.com | 833-OEA-KAUP 632-5287 OEAK-BROC-2021-04-29 ABOUT US OE Attachments is proud to offer a new product line of attachments through its exclusive North American partnership with German manufacturer Kaup. Producing over 40,000 attachments every year, Kaup is one of Europe’s leading manufacturers in forklift attachments such as sideshifters, fork positioners, rotators, and more. All OEA Kaup attachments are modular and designed to work together. TABLE OF CONTENTS Fork Clamps 4 3 Rotators 6 Multi-Pallet Handlers 8 Appliance & Carton Clamps 10 Telescopic Forks 12 Rotating Roll Clamps 14 Push Pulls 16 Sideshifters 18 Fork Positioners 20 Fork Variety 22 Load Backrest 24 OEAK-BROC-2021-04-29 OEattachments.com | 833-OEA-KAUP 632-5287 FORK CLAMPS OEA Kaup Fork Clamps High-Visibility Fork Clamps can be used for transporting palletized goods as well as for clamping. OEA Kaup’s optimized design ensures superior visibility for the driver and allows quick and precise operation of the clamp. The compact dimensions provide stability, and the robust sliding profile decreases wear and tear. Customization of the opening ranges, frame widths, fork types, and sizes adds new levels of versatility. HIGH VISIBILITY OEA Kaup Clamps set high standards with regards to visibility. The optimized design ensures excellent visibility for the driver and allows quick and precise operation of the clamp guaranteeing high working performance and reducing the risk of accidents significantly. HANDLING WITH OEA KAUP CLAMPS OEA Kaup Clamps can be supplied with sideshift function which simplifies operation for the truck driver.