(Paralichthys Lethostigma) in the Galveston Bay Estuary, TX
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Winter Flounder Pseudopleuronectes Americanus Stock Enhancement in New Hampshire: Developing Optimal Release Strategies
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2002 Winter flounder Pseudopleuronectes americanus stock enhancement in New Hampshire: Developing optimal release strategies Elizabeth Alden Fairchild University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation Fairchild, Elizabeth Alden, "Winter flounder Pseudopleuronectes americanus stock enhancement in New Hampshire: Developing optimal release strategies" (2002). Doctoral Dissertations. 62. https://scholars.unh.edu/dissertation/62 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. -
Schedule Onlinepdf
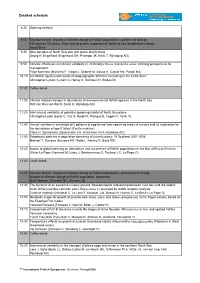
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

NHBSS 034 1G Wongratana R
NAT. NAT. HIST. BULL. SIAM So c. 34 (1) :65 ・70 ,1986 RECORD OF AMBICOLORATION IN CYNOGLOSSUS (PISCES : CYNOGLOSSIDAE) FROM THAILAND Thosaporn Thosaporn Wongratana * ABSTRACT An almost ambico10rate “Four- Ii ned tongu e- sole" ,Cynoglossus bilinealus (La cepede) , is is reported from Thailand. It is presumab1y the first record for the genus. Except for most of of the head on the blind side and its.corresponding finrays ,which are pale as in normal specimens ,the body and fins are pigmented. The norma Jl y cycloid scales on the blind side in in the pigmented area are who Jl y replaced by ctenoid scales ,but those on the unpigmented part part on the head are cyc10id. The latera1line sca1es of the pigmented area on the blind side are are cycloid. The pelvic fins are entirely separated from the anal fin by the absence of membrane. membrane. No other major externa1 anomaly is found. PREVIOUS ACCOUNTS Abnormalities Abnormalities in coloration 釘 e more common among members of the order Pleuronectiformes Pleuronectiformes than in any other group of fishes. Other anomalies occasionally in those those fishes are a hooked dorsal fin , incomplete eye migration and side reversa l. Abnormal pigmentation in flatfish is divided into three main types: ambicoloration , albinism ,and xanthochromism (DEVEEN , 1969; COLMAN ,1972 勾). P 町 “tia 剖10 町Ir incomplete ambic ∞010 町r羽 ion is mor 問ec ∞ommon than trunk pigmentation , nearly complete amb 凶ic ∞0- lor 問at “ion and complete ambic ∞010 町ra 創ti 拘on (υJONES & MENON , 1950). NORMAN'S (1 934) previous previous explanation of this phenomenon ,later accepted by many authors ,was that “..訓 nbicoloration merely represents variation in the direction of the original bilateral symmetrical symmetrical condition of the ancester of flatfishes." It is also regularly observed that that wholly ambicolorate fishes normally display a higher degree of symmetry than normal normal specimens in pigmentation ,scales , paired fins and final position of the eyes (NORMAN , 1934; COLMAN , 1972). -

For Summer Flounder Is Defined As
FISHERY MANAGEMENT PLAN FOR THE SUMMER FLOUNDER FISHERY October 1987 Mid-Atlantic Fishery Management Council in cooperation with the National Marine Fisheries Service, the New England Fishery Management Council, and the South Atlantic Fishery Management Council Draft adopted by MAFMC: 29 October 1987 Final adopted by MAFMC: 16 April1988 Final approved by NOAA: 19 September 1988 3.14.89 FISHERY MANAGEMENT PLAN FOR THE SUMMER FLOUNDER FISHERY October 1987 Mid-Atlantic Fishery Management Council in cooperation with the National Marine Fisheries Service, the New England Fishery Management Council, and the South Atlantic Fishery Management Council See page 2 for a discussion of Amendment 1 to the FMP. Draft adopted by MAFMC: 21 October 1187 final adopted by MAFMC: 16 April1988 final approved by NOAA: 19 September 1988 1 2.27 91 THIS DOCUMENT IS THE SUMMER FLOUNDER FISHERY MANAGEMENT PLAN AS ADOPTED BY THE COUNCIL AND APPROVED BY THE NATIONAL MARINE FISHERIES SERVICE. THE REGULATIONS IN APPENDIX 6 (BLUE PAPER) ARE THE REGULATIONS CONTROLLING THE FISHERY AS OF THE DATE OF THIS PRINTING (27 FEBRUARY 1991). READERS SHOULD BE AWARE THAT THE COUNCIL ADOPTED AMENDMENT 1 TO THE FMP ON 31 OCTOBER 1990 TO DEFINE OVERFISHING AS REQUIRED BY 50 CFR 602 AND TO IMPOSE A 5.5" (DIAMOND MESH) AND 6" (SQUARE MESH) MINIMUM NET MESH IN THE TRAWL FISHERY. ON 15 FEBRUARY 1991 NMFS APPROVED THE OVERFISHING DEFINITION AND DISAPPROVED THE MINIMUM NET MESH. OVERFISHING FOR SUMMER FLOUNDER IS DEFINED AS FISHING IN EXCESS OF THE FMAX LEVEL. THIS ACTION DID NOT CHANGE THE REGULATIONS DISCUSSED ABOVE. 2 27.91 2 2. -

Combined Effects of Turbulence and Salinity on Growth, Survival, and Whole-Body Osmolality of Larval Southern Flounder
JOURNAL OF THE Vol. 37, No. 4 WORLD AQUACULTURE SOCIETY December, 2006 Combined Effects of Turbulence and Salinity on Growth, Survival, and Whole-body Osmolality of Larval Southern Flounder ADAM MANGINO JR. AND WADE O. WATANABE1 University of North Carolina Wilmington, Center for Marine Science, 7205 Wrightsville Avenue, Wilmington, North Carolina 28403 USA Abstract The southern flounder (Paralichthys lethostigma) is a commercially important marine flatfish from the southeastern Atlantic and Gulf Coasts of the USA and an attractive candidate for aquaculture. Hatchery methods are relatively well developed for southern flounder; however, knowledge of the optimum environmental conditions for culturing the larval stages is needed to make these technologies more cost effective. The objectives of this study were to determine the effects of water turbulence (as controlled by varying rates of diffused aeration) on growth, survival, and whole-body osmolality of larval southern flounder from hatching through day 16 posthatching (d16ph). Embryos were stocked into black 15-L cylindrical tanks under four turbulence levels (20, 90, 170, and 250 mL/min of diffused aeration) and two salinities (24 and 35 ppt) in a 4 3 2 factorial design. Larvae were provided with enriched s-type rotifers from d2ph at a density of 10 individuals/mL. Temperature was 19 C, light intensity was 390 lx, and photoperiod was 18 L:6 D. Significant (P , 0.05) effects of turbulence on growth (notochord length [NL], wet weight, and dry weight) were observed. On d16ph, NL (mm) increased with decreasing turbulence level and was significantly greater at 20 mL/min (64.2) and 90 mL/min (58.2) than at 170 mL/min (56.3) and 250 mL/min (57.2). -

Distribution and Abundance of Pleuronectiformes Larvae Off Southeastern Brazil
BRAZILIAN JOURNAL OF OCEANOGRAPHY, 62(1):23-34, 2014 DISTRIBUTION AND ABUNDANCE OF PLEURONECTIFORMES LARVAE OFF SOUTHEASTERN BRAZIL Camilla Nunes Garbini*, Maria de Lourdes Zani-Teixeira , Márcio Hidekazu Ohkawara and Mario Katsuragawa Instituto Oceanográfico da Universidade de São Paulo (Praça do Oceanográfico, 191, 05508-120 São Paulo, SP, Brasil) *Corresponding author: [email protected] http://dx.doi.org/10.1590/S1679-87592014051706201 ABSTRACT The objective of this study was the description of the composition, abundance and density in horizontal and vertical distribution of Pleuronectiformes larvae on the southeastern Brazilian continental shelf. The samples were collected with bongo nets and a Multi Plankton Sampler (MPS), both in summer and winter 2002. A total of 352 flatfishes larvae were collected in summer and 343 in winter, representing three families and a total of 13 taxa: Paralichthyidae ( Citharichthys cornutus, C. spilopterus, Citharichthys sp ., Cyclopsetta chittendeni, Syacium spp ., Etropus spp . and Paralichthys spp .), Bothidae ( Bothus ocellatus and Monolene antillarum ) and Cynoglossidae ( Symphurus trewavasae, S. jenynsi, S. plagusia and S. ginsburgi ). The most abundant taxa were Etropus spp ., Syacium spp . and Bothus ocellatus . Etropus spp . occurred mainly as far out as the 200 m isobath and Syacium spp . from 100 m. B. ocellatus was present mainly in the oceanic zone between Ubatuba and Rio de Janeiro as from the 200 m isobath. The greatest average densities of these species occurred in the strata from 0 to 20 m depth in summer and between 20 and 40 m in winter. RESUMO O objetivo deste estudo foi descrever a composição, abundância, densidade, distribuição horizontal e vertical das larvas de Pleuronectiformes ao longo da plataforma continental Sudeste brasileira. -

Locus Number Estimation of MHC Class II B in Stone Flounder and Japanese Flounder
Int. J. Mol. Sci. 2015, 16, 6000-6017; doi:10.3390/ijms16036000 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article Locus Number Estimation of MHC Class II B in Stone Flounder and Japanese Flounder Jiajun Jiang †, Chunmei Li †, Quanqi Zhang and Xubo Wang * Key Laboratory of Marine Genetics and Breeding (Ocean University of China), Ministry of Education, Qingdao 266003, China; E-Mails: [email protected] (J.J.); [email protected] (C.L.); [email protected] (Q.Z.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +86-532-8203-1986. Academic Editor: Ritva Tikkanen Received: 2 December 2014 / Accepted: 25 December 2014 / Published: 13 March 2015 Abstract: Members of major histocompatibility complex (MHC) family are important in immune systems. Great efforts have been made to reveal their complicated gene structures. But many existing studies focus on partial sequences of MHC genes. In this study, by gene cloning and sequencing, we identified cDNA sequences and DNA sequences of the MHC class II B in two flatfishes, stone flounder (Kareius bicoloratus) and homozygous diploid Japanese flounder (Paralichthys olivaceus). Eleven cDNA sequences were acquired from eight stone flounder individuals, and most of the polymorphic sites distributed in exons 2 and 3. Twenty-eight alleles were identified from the DNA fragments in these eight individuals. It could be deduced from their Bayesian inference phylogenetic tree that at least four loci of MHC class II B exist in stone flounder. The detailed whole-length DNA sequences in one individual were analyzed, revealing that the intron length varied among different loci. -

Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2016 Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes Christi Linardich Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biodiversity Commons, Biology Commons, Environmental Health and Protection Commons, and the Marine Biology Commons Recommended Citation Linardich, Christi. "Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes" (2016). Master of Science (MS), Thesis, Biological Sciences, Old Dominion University, DOI: 10.25777/hydh-jp82 https://digitalcommons.odu.edu/biology_etds/13 This Thesis is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES by Christi Linardich B.A. December 2006, Florida Gulf Coast University A Thesis Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE BIOLOGY OLD DOMINION UNIVERSITY August 2016 Approved by: Kent E. Carpenter (Advisor) Beth Polidoro (Member) Holly Gaff (Member) ABSTRACT HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES Christi Linardich Old Dominion University, 2016 Advisor: Dr. Kent E. Carpenter Understanding the status of species is important for allocation of resources to redress biodiversity loss. -

Southern Flounder
REFERENCECOPY Do Not Remove from the Library U. S. Fish and Wildlife Service Biological Report, 82(11.30) lUatlona' vveTlanasKeSemr TR EL-82-4 April. 1985 700 Cajun Dome Boule\lard Lafayette, Louisiana 7r1536 Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (Gulf of Mexico) SOUTHERN FLOUNDER Coastal Ecology Group Fish and Wildlife Service Waterways Experiment Station U.S. Department of the Interior U.S. Army Corps of Engineers This is one of the first reports to be publ ished in the new "Biological Report'' series. This technical report series, publ ished by the Research and Development branch of the U.S. Fish and Wildlife Service, replaces the "FWS/OBS1I series published from 1976 to September 1984. The Biolog- ical Report series is designed for the rapid publ ication of reports with an application orientation, and it continues the focus of the FWS/OBS series on resource management issues and fish and wild1ife needs. Biological Report 82(11.30) TR EL-82-4 April 1985 Species Profiles: Life Hi stories and Envi ronlnental Requirements of Coastal Fishes and Invertebrates (Gulf of Mexico) SOUTHERN FLOUNDER Roland E. Reagan, Jr. and William M. Wingo Department of Wildlife and Fisheries 136 Dorrnan Hal 1 Mississippi State University Missi ssi ppi State, MS 39 762 Project Manager Larry Shanks Project Officer John Parsons National Coastal Ecosystems Team U.S. Fish and Wildlife Service 1010 Gause Boulevard Sl idel 1 , LA 70458 Performed for Coastal Ecol ogy Group U.S. Army Corps of Engineers Waterways Experiment Station Vicksburg, MS 39180 and National Coastal Ecosystems Team Division of Biological Services Research and Development Fi sh and Wi1 dli fe Servi ce U.S. -

Fish Study Cover 3
Putnam County Environmental Council ! !"#"$%&%#'("#)(*%+',-"'.,#(,/( '0%(1.+0(2,345"'.,#+(,/(6.57%-( 63-.#$+("#)('0%(!.))5%("#)(8,9%-( :;<5"9"0"(*.7%-=(15,-.)"=(>6?( ( *,@(*A(8%9.+(BBB=(!A?A=(2ACA6A( MANAGEMENT AND RESTORATION OF THE FISH POPULATIONS OF SILVER SPRINGS AND THE MIDDLE AND LOWER OCKLAWAHA RIVER, FLORIDA, USA A Special Report for The Putnam County Environmental Council Funded by a Grant from the Felburn Foundation By Roy R. “Robin” Lewis III, M.A., P.W.S. Certified Professional Wetland Scientist and Certified Senior Ecologist May 14, 2012 Cover photograph: Longnose Gar, Lepisosteus osseus, in Silver Springs, Underwater Photograph by Peter Butt, KARST Environmental ACKNOWLEDGEMENTS The author wishes to thank all those who reviewed and commented on the numerous drafts of this document, including Paul Nosca, Michael Woodward, Curtis Kruer and Sandy Kokernoot. All conclusions, however, remain the responsibility of the author. CITATION The suggested citation for this report is: LEWIS, RR. 2012. MANAGEMENT AND RESTORATION OF THE FISH POPULATIONS OF SILVER SPRINGS AND THE MIDDLE AND LOWER OCKLAWAHA RIVER, FLORIDA, USA. Putnam County Environmental Council, Interlachen, Florida. 27 p + append. Additional copies of this document can be downloaded from the PCEC website at www.pcecweb.org. i EXECUTIVE SUMMARY Sixty‐nine (69) species of native fish have been documented to have utilized Silver Springs, Silver River and the Upper, Middle and Lower Ocklawaha River for the period of record. Fifty‐nine of these are freshwater fish species and ten are native migratory species using marine, estuarine and freshwater habitats during their life history. These include striped bass, American eel, American shad, hickory shad, hogchoker, striped mullet, channel and white catfish, needlefish and southern flounder. -

16-Astarloa 302.Indd
Notes ichtyologiques / Ichthyological notes First report of a totally ambicoloured Patagonian flounderParalichthys patagonicus (Paralichthyidae) with dorsal fin anomalies by Juan M. DÍAZ DE ASTARLOA (1), Rita RICO (2) & Marcelo ACHA (1, 2) RÉSUMÉ. - Premier signalement d’un Paralichthys patagonicus RESULTS totalement ambicoloré, avec des anomalies de la nageoire dorsale. The flatfish has the typical pigmentation developed on the ocu- L’exemplaire, capturé dans les eaux des côtes d’Argentine au large de Necochea (38º37’S-58º50’W), est entièrement ambicoloré, lar side characteristic for the species. It differs from other speci- et présente aussi un développement anormal de la nageoire dorsale mens in that it is also virtually totally pigmented on the blind side et une migration incomplète de l’œil droit. Il s’agit ici du troisième (Fig. 1A). The entire blind (right) side is uniformly dark brown, signalement du phénomène d’ambicoloration chez une espèce de Paralichthys dans l’Atlantique sud-ouest. Key words. - Paralichthyidae - Paralichthys patagonicus - ASW - Argentine coast - Ambicoloration - Head anomalies. A typical anomaly of flatfishes is malpigmentation, which is characterized by either a deficiency of pigment cells on portions of the ocular side (albinism, pesudoalbinism, or hypomelanism), or the presence of dark pigmentation on the normally light-coloured underside of the fish, also called ambicoloration (Bolker and Hill, 2000). In May 2003, one totally ambicoloured and anomalous speci- men of the Patagonian flounderParalichthys patagonicus Jordan, in Jordan and Goss, 1889 was caught by a commercial bottom- trawler off Necochea (38°37’S-58°50’W), Argentina, at 31 m depth. Paralichthys patagonicus is one of seven paralichthyid flounders known from southwestern Atlantic waters (Díaz de Astarloa, 1994). -

Wasted Resources: Bycatch and Discards in U. S. Fisheries
Wasted Resources: Bycatch and discards in U. S. Fisheries by J. M. Harrington, MRAG Americas, Inc. R. A. Myers, Dalhousie University A. A. Rosenberg, University of New Hampshire Prepared by MRAG Americas, Inc. For Oceana July 2005 TABLE OF CONTENTS ACKNOWLEDGEMENTS 7 NATIONAL OVERVIEW 9 Introduction 9 Methodology 11 Discarded Bycatch Estimates for the 27 Major Fisheries in the U.S. 12 Recommendations 17 Definitions of Key Terms Used in the Report 19 Acronyms and Abbreviations Used in the Report 20 NORTHEAST 25 Northeast Groundfish Fishery 27 Target landings 28 Regulations 30 Discards 32 Squid, Mackerel and Butterfish Fishery 41 Target landings 42 Regulations 44 Discards 44 Monkfish Fishery 53 Target landings 53 Regulations 54 Discards 55 Summer Flounder, Scup, and Black Sea Bass Fishery 59 Target landings 59 Regulations 60 Discards 61 Spiny Dogfish Fishery 69 Target landings 69 Regulations 70 Discards 70 Atlantic Surf Clam and Ocean Quahog Fishery 75 Target landings 75 Regulations 76 Discards 76 Atlantic Sea Scallop Fishery 79 Target landings 79 Regulations 80 Discards 81 Atlantic Sea Herring Fishery 85 Target landings 85 Regulations 86 Discards 87 Northern Golden Tilefish Fishery 93 Target landings 93 Regulations 94 Discards 94 Atlantic Bluefish Fishery 97 Target landings 97 Regulations 98 Discards 98 Deep Sea Red Crab Fishery 101 Target landings 101 Regulations 101 Discards 102 SOUTHEAST 103 Shrimp Fishery of the South Atlantic 105 Target landings 105 Regulations 106 Discards 107 Snapper and Grouper of the South Atlantic 111 Target