A Systematic Review of the Effectiveness of Antianxiety and Antidepressive Agents for Functional Dyspepsia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Developments in Prokinetic Therapy for Gastric Motility Disorders
REVIEW published: 24 August 2021 doi: 10.3389/fphar.2021.711500 New Developments in Prokinetic Therapy for Gastric Motility Disorders Michael Camilleri* and Jessica Atieh Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, United States Prokinetic agents amplify and coordinate the gastrointestinal muscular contractions to facilitate the transit of intra-luminal content. Following the institution of dietary recommendations, prokinetics are the first medications whose goal is to improve gastric emptying and relieve symptoms of gastroparesis. The recommended use of metoclopramide, the only currently approved medication for gastroparesis in the United States, is for a duration of less than 3 months, due to the risk of reversible or irreversible extrapyramidal tremors. Domperidone, a dopamine D2 receptor antagonist, is available for prescription through the FDA’s program for Expanded Access to Investigational Drugs. Macrolides are used off label and are associated with tachyphylaxis and variable duration of efficacy. Aprepitant relieves some symptoms of gastroparesis. There are newer agents in the pipeline targeting diverse gastric (fundic, antral and pyloric) motor functions, including novel serotonergic 5-HT4 agonists, dopaminergic D2/3 antagonists, neurokinin NK1 antagonists, and ghrelin agonist. Novel Edited by: targets with potential to improve gastric motor functions include the pylorus, macrophage/ Jan Tack, inflammatory function, oxidative -

Prokinetics and Ghrelin for the Management of Cancer Cachexia Syndrome
85 Review Article Prokinetics and ghrelin for the management of cancer cachexia syndrome Jimi S. Malik, Sriram Yennurajalingam Department of Palliative Care, Rehabilitation and Integrative Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA Contributions: (I) Conception and design: All authors; (II) Administrative support: S Yennurajalingam; (III) Provision of study materials or patients: S Yennurajalingam; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Sriram Yennurajalingam, MD. Department of Palliative Care, Rehabilitation, and Integrative Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1414, Houston, TX 77030, USA. Email: [email protected]. Abstract: Cancer cachexia (CC) is one of the most distressing syndromes for both patients and their families. CC can have an impact on patient reported quality of life and overall survival. It is often associated with symptoms such as fatigue, depressed mood, early satiety, and anorexia. Prokinetic agents have been found to improve chronic nausea and early satiety associated with CC. Among the prokinetic agents, metoclopramide is one of the best studied medications. The role of the other prokinetic agents, such as domperidone, erythromycin, haloperidol, levosulpiride, tegaserod, cisapride, mosapride, renzapride, and prucalopride is unclear for use in cachectic cancer patients due to their side effect profile and limited efficacy studies in cancer patients. There has been an increased interest in the use of ghrelin-receptor agonists for the treatment of CC. Anamorelin HCl is a highly selective, novel ghrelin receptor agonist. -

(Or) Aravind Doki
Available Online through www.ijpbs.com (or) www.ijpbsonline.com IJPBS |Volume 3| Issue 3 |JUL-SEP|2013|152-161 Research Article Pharmaceutical Sciences METHOD DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR SIMULTANEOUS ESTIMATION OF CLIDINIUM BROMIDE, CHLORDIAZEPOXIDE AND DICYCLOMINE HYDROCHLORIDE IN BULK AND COMBINED TABLET DOSAGE FORMS Aravind.Doki* and Kamarapu.SK Department of Pharmaceutical Analysis, Sri Shivani College Of Pharmacy, Mulugu Road, Warangal, Andrapradesh, India, 506001. *Corresponding Author Email: [email protected] ABSTRACT The study describes method development and subsequent validation of RP-HPLC method for simultaneous estimation of Clidinium bromide (CDB), Chlordiazepoxide (CDZ) and Dicyclomine hydrochloride (DICY) in bulk and combined tablet dosage forms. Chromatographic separation was achieved on a Kromasil C18 (250 mm × 4.6 mm id, 5µm) column using a mobile phase ratio consisting of (40:30:30) Methanol: Acetonitrile: Potassium di hydrogen phosphate buffer (0.05M, PH 4.0 adjusting with 0.5% Ortho phosphoric acid) at flow rate 1.0 ml/min. The detection wavelength is 270 nm. The retention times of Clidinium bromide, Chlordiazepoxide and Dicyclomine hydrochloride were found to be 7.457 min, 4.400 min and 3.397 min respectively. The developed method was validated as per ICH guidelines using the parameters such as accuracy, precision, linearity, LOD, LOQ, ruggedness and robustness. The developed and validated method was successfully used for the quantitative analysis of Clidinium bromide, Chlordiazepoxide and Dicyclomine hydrochloride in bulk and combined tablet dosage forms. KEY WORDS Clidinium bromide, Chlordiazepoxide and Dicyclomine hydrochloride, Normaxin tablet dosage forms, HPLC, Method validation. INTRODUCTION hydrochloride (1, 1-bicyclohexyl-1-carboxilicacid-2- Clidinium bromide (3-[(2-hydroxy-2, [diethyl amino] ethyl ester) is an anticholinergic drug 2diphenylacetyl)-oxy]-1-methyl-1-azoniabicylo- (tertiary amine). -

Muscarinic Acetylcholine Receptor
mAChR Muscarinic acetylcholine receptor mAChRs (muscarinic acetylcholine receptors) are acetylcholine receptors that form G protein-receptor complexes in the cell membranes of certainneurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibersin the parasympathetic nervous system. mAChRs are named as such because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpineand scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists. Acetylcholine (ACh) is a neurotransmitter found extensively in the brain and the autonomic ganglia. www.MedChemExpress.com 1 mAChR Inhibitors & Modulators (+)-Cevimeline hydrochloride hemihydrate (-)-Cevimeline hydrochloride hemihydrate Cat. No.: HY-76772A Cat. No.: HY-76772B Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic receptor agonist, is a candidate therapeutic drug for receptor agonist, is a candidate therapeutic drug for xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR The general pharmacol. properties of this drug on the The general pharmacol. properties of this drug on the gastrointestinal, urinary, and reproductive systems and other… gastrointestinal, urinary, and reproductive systems and other… Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10mM x 1mL in DMSO, Size: 10mM x 1mL in DMSO, 1 mg, 5 mg 1 mg, 5 mg AC260584 Aclidinium Bromide Cat. No.: HY-100336 (LAS 34273; LAS-W 330) Cat. -

77Da2550569e5553dc05b76601
www.ijpsonline.com 2003;13:121-7. RM, Medina-Hernandez MJ. Determination of anticonvulsant drugs in 10. French WN, Matsui FF, Smith SJ. Determination of major impurity pharmaceutical preparations by micellar liquid chromatography. J Liq in chlordiazepoxide formulations and drug substance. J Pharm Sci Chromatogr Related Techno 2004;27:153-70. 2006;64:1545-7. 15. Toral MI, Richter P, Lara N, Jaque P, Soto C, Saavedra M. 11. Stahlmann S, Karl-Artur K. Analysis of impurities by high-performance Simultaneous determination of chlordiazepoxide and clidinium bromide thin-layer chromatography with fourier transform infrared spectroscopy in pharmaceutical formulations by derivative spectrophotometry. Int J and UV absorbance detection in situ measurement: chlordiazepoxide in Pharm 1999;189:67-74. bulk powder and in tablets. J Chromatogr-A 1998;13:145-52. 16. Beckett AH, Stenlake JB. Practice Pharmaceutical Chemistry; 4th ed. 12. Saudagar RB, Saraf S. Spectrophotometric determination of Part II. New Delhi: CBS Publishers; 1997. p. 285. chlordiazepoxide and trifluoperazine hydrochloride from combined dosage form. Indian J Pharm Sci 2007;69:149-52. Accepted 13 August 2009 13. Davidson AG. Assay of chlordiazepoxide and demoxepam in Revised 20 May 2009 chlordiazepoxide formulations by difference spectrophotometry. J Received 24 June 2008 Pharm Sci 1984;73:55-8. 14. Cholbi-Cholbi MF, Martínez-Pla JJ, Sagrado S, Villanueva-Camanas Indian J. Pharm. Sci., 2009, 71 (4): 468-472 Spectrophotometric and Chromatographic Simultaneous Estimation of Amitriptyline Hydrochloride and Chlordiazepoxide in Tablet Dosage Forms SEJAL PATEL* AND N. J. PATEL S. K. Patel College of Pharmaceutical Education and Research, Department of Pharmaceutical Chemistry, Ganpat University, Kherva, Mehsana-382711, Gujarat, India Patel and Patel, et al.: Simultaneous Estimation of Amitriptyline Hydrochloride and Chlordiazepoxide A binary mixture of amitriptyline HCl and chlordiazepoxide was determined by three different methods. -

ST Louis Formulary
Learn About Your Formulary Your drug list, also called a formulary, is the list of drugs covered by St. Louis County Department of Health. Your plan with the St. Louis County Department of Health has specific guidelines you must follow to get prescription drug coverage. You must choose generic medications when they are Many drug companies offer programs to help people available. Generic medications have the same ingredients afford their drugs. We encourage you to ask your doctor as brand drugs, at lower prices. about manufacturer assistance programs. You must fill your prescription at participating pharmacies Drugs are listed on the formulary in alphabetical order by within the St. Louis area: name in the appropriate therapeutic category and class. > CVS > The Medicine Shoppe > Walmart > Schnucks Medications on the High Priority Drug List are covered for > Sam's Club > Beverly Hills Pharmacy ONLY two 30-day fills per year. > Dierbergs > If you are prescribed a High Priority Drug, contact the drug maker to request assistance in getting your medication at reduced or no cost. You must get your prescriptions from Department of > If you do not take this step, you will pay 100% of the Health Physicians discounted drug cost after getting two 30-day fills. > If a medication is prescribed by a non-panel doctor, a > The High Priority Drug List includes: special review is required and can be requested at the • Diabetes drugs: Lantus, Novolog and Novolin number below. • Asthma Inhalers: Atrovent, Spiriva, Ventolin, Proair, Respiclick, Symbicort, Advair, Flovent and Proventil Online Tools Who can I call if I have a question? Our Customer Service Center is available 24 hours a day, 7 Access your pharmacy beneft information through our days a week, 365 days a year to help answer any questions or portal, Members.EnvolveRx.com, and mobile app, concerns you have about your pharmacy benefit. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Clidinium Bromide
Clidinium Bromide sc-207449 Material Safety Data Sheet Hazard Alert Code Key: EXTREME HIGH MODERATE LOW Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Clidinium Bromide STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH1 HAZARD INSTABILITY0 SUPPLIER Company: Santa Cruz Biotechnology, Inc. Address: 2145 Delaware Ave Santa Cruz, CA 95060 Telephone: 800.457.3801 or 831.457.3800 Emergency Tel: CHEMWATCH: From within the US and Canada: 877-715-9305 Emergency Tel: From outside the US and Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 PRODUCT USE Quaternary ammonium antimuscarinic agent with peripheral actions similar to those of atropine. For the symptomatic relief of peptic ulcer and other gastrointestinal disorders. Taken by mouth. SYNONYMS C22-H26-Br-N-O3, C22-H26-Br-N-O3, "1-azoniabicyclo(2.2.2)octane, 3-[(hydroxydiphenylacetyl)oxy]-1-methyl-, ", "1-azoniabicyclo(2.2.2)octane, 3-[(hydroxydiphenylacetyl)oxy]-1-methyl-, ", bromide, "benzilic acid, ester with 3-hydroxy- 1-methylquinuclidinium", "benzilic acid, ester with 3-hydroxy-1-methylquinuclidinium", "3-(benziloyloxy)-1-methylquinuclidinium bromide", "3-(benziloyloxy)-1-methylquinuclidinium bromide", "3-hydroxy-1-methylquinuclidinium bromide benzilate", "3-hydroxy- 1-methylquinuclidinium bromide benzilate", "1-methyl-3-benziloxyloxy-quinuclidinium bromide", "1-methyl-3-benziloxyloxy-quinuclidinium bromide", "quinuclidinium, 3-hydroxy-1-methyl-bromide, benzilate", "quinuclidinium, 3-hydroxy-1-methyl-bromide, benzilate", "quinuclidinol methylbromide, benzilate", Apo-Chlorax, Chlorax, Clipoxide, Corium, Librax, Libraxin, Quarzan, "Quarzan bromide", RO-2-3773, RO-2-3773, "quaternary ammonium antimuscarinic/ anticholinergic" Section 2 - HAZARDS IDENTIFICATION CANADIAN WHMIS SYMBOLS 1 of 13 Clidinium Bromide sc-207449 Material Safety Data Sheet Hazard Alert Code Key: EXTREME HIGH MODERATE LOW EMERGENCY OVERVIEW RISK Harmful if swallowed. -

Dizziness/Balance Evaluation Patient Instructions You Are Scheduled for a Test of Your Balance System
Dizziness/Balance Evaluation Patient Instructions You are scheduled for a test of your balance system. There are a few things you should know prior to your appointment. MEDICATIONS Certain medications affect the test results. Below is a partial list of medications that should not be taken for 48 hours prior to the test. Ask your doctor if you have concerns about discontinuing your medications. The list below is a partial list with generic name first and trademark name bolded in parenthesis. • Alcohol - beer, wine, liquor, cough medicine • Medications commonly used to reduce dizziness and nausea - Meclizine (Antivert, Bonnine, Ru-Vert-M), Hydroxyzine (Atarax, Vistaril), Lorazepam (Ativan), Diphenhydramine (Benadryl), Prochlorperazine (Compazine), Dimenhydrinate (Dramamine), Clonazepam (Klonipin), Cyclizine (Marezine), Promethazine (Phenergan), Trimethobenzamine (Tigan), Diazepam (Valium), Nearly all motion sickness patches or medications. OTHER MEDICATIONS • Analgesics/Narcotics - Codeine, Demerol, Phenaphen, Tylenol with Codeine, Percocet, Darvocet • Anti-histamines - Fexofenadine (Allegra), Loratadine (Claritin, Alavert), Chlorpheniramine (Chlor-Trimeton, Efidac, Teldrin), Brompheniramine (Dimetane), Clemastine (Tavist), Cetirizine (Zyrtec), nearly all-over-the-counter allergy or flu/cold medicines • Sedatives - Flurazepam (Dalmane), Triazolam (Halcion), Pentobarbital (Nembutal), Temazepam (Restoril), Secobarbital (Seconal), among others. • Tranquilizers/Anti-anxiety medications - Chlordiazepoxide hydrochloride and clidinium bromide (Librax), -

Federal Register/Vol. 77, No. 115/Thursday, June 14, 2012
Federal Register / Vol. 77, No. 115 / Thursday, June 14, 2012 / Notices 35691 TABLE 1—LIST OF SAFETY AND EFFECTIVENESS SUMMARIES FOR APPROVED PMAS MADE AVAILABLE FROM JANUARY 1, 2012, THROUGH MARCH 31, 2012—Continued PMA No., Docket No. Applicant Trade name Approval date P060008.S046, FDA–2012–M–0210 ... Boston Scientific Corp ......................... TAXUS Liberte´ Paclitaxel-Eluting Cor- February 22, 2012. onary Stent System (Monorail and Over-The-Wire Delivery Systems). P030025.S086, FDA–2012–M–0209 ... Boston Scientific Corp ......................... TAXUS Express2 Paclitaxel-Eluting February 22, 2012. Coronary Stent System (Monorail and Over-The-Wire Delivery Sys- tems). P110023, FDA–2012–M–0221 ............ ev3, Inc ................................................ Everflex Self-Expanding Peripheral March 7, 2012. Stent System (Everflex). P070004, FDA–2012–M–0250............ Sientra, Inc.......................................... SIENTRA Silicone Gel Breast Im- March 9, 2012. plants. II. Electronic Access LOCATION: The meeting will be held at submissions. In the process of Persons with access to the Internet the FDA White Oak Campus, 10903 considering these changes, FDA has may obtain the documents at http:// New Hampshire Ave., Bldg. 31 previously made available for comment www.fda.gov/MedicalDevices/ Conference Center, Great Room 1503, versions of documents that support ProductsandMedicalProcedures/ Silver Spring, MD 20993. The following making regulatory submissions in DeviceApprovalsandClearances/ link contains public meeting attendee electronic format using the (eCTD) information as well as frequently asked PMAApprovals/default.htm and http:// specifications. These draft documents questions and answers regarding public www.fda.gov/MedicalDevices/ represented FDA’s major updates to meetings at White Oak: http:// ProductsandMedicalProcedures/ Module 1 of the eCTD based on www.fda.gov/AboutFDA/ DeviceApprovalsandClearances/ previous comments. -
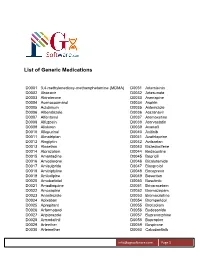
List of Generic Medications
List of Generic Medications D0001 3,4-methylenedioxy-methamphetamine (MDMA) D0031 Artemisinin D0002 Abacavir D0032 Artesunate D0003 Abiraterone D0033 Asenapine D0004 Acenocoumarol D0034 Aspirin D0005 Aclidinium D0035 Astemizole D0006 Albendazole D0036 Atazanavir D0007 Alfentanyl D0037 Atomoxetine D0008 Alfuzosin D0038 Atorvastatin D0009 Aliskiren D0039 Avanafil D0010 Allopurinol D0040 Axitinib D0011 Almotriptan D0041 Azathioprine D0012 Alogliptin D0042 Azilsartan D0013 Alosetron D0043 Bazedoxifene D0014 Alprazolam D0044 Bedaquiline D0015 Amantadine D0045 Bepridil D0016 Amiodarone D0046 Bicalutamide D0017 Amisulpride D0047 Bisoprolol D0018 Amitriptyline D0048 Boceprevir D0019 Amlodipine D0049 Bosentan D0020 Amobarbital D0050 Bosutinib D0021 Amodiaquine D0051 Brivaracetam D0022 Amoxapine D0052 Bromazepam D0023 Anastrozole D0053 Bromocriptine D0024 Apixaban D0054 Bromperidol D0025 Aprepitant D0055 Brotizolam D0026 Arformoterol D0056 Budesonide D0027 Aripiprazole D0057 Buprenorphine D0028 Armodafinil D0058 Bupropion D0029 Arteether D0059 Buspirone D0030 Artemether D0060 Cabozantinib [email protected] Page 1 List of Generic Medications D0061 Canagliflozin D0091 Clofarabine D0062 Cannabidiol (CBD) D0092 Clofibrate D0063 Cannabinol (CBN) D0093 Clomipramine D0064 Captopril D0094 Clonazepam D0065 Carbamazepine D0095 Clonidine D0066 Carisoprodol D0096 Clopidogrel D0067 Carmustine D0097 Clorazepate D0068 Carvedilol D0098 Clozapine D0069 Celecoxib D0099 Cocaine D0070 Ceritinib D0100 Codeine D0071 Cerivastatin D0101 Colchicine D0072 Cetirizine -

Gastroparesis: 2014
GASTROINTESTINAL MOTILITY AND FUNCTIONAL BOWEL DISORDERS, SERIES #1 Richard W. McCallum, MD, FACP, FRACP (Aust), FACG Status of Pharmacologic Management of Gastroparesis: 2014 Richard W. McCallum Joseph Sunny, Jr. Gastroparesis is characterized by delayed gastric emptying without mechanical obstruction of the gastric outlet or small intestine. The main etiologies are diabetes, idiopathic and post- gastric and esophageal surgical settings. The management of gastroparesis is challenging due to a limited number of medications and patients often have symptoms, which are refractory to available medications. This article reviews current treatment options for gastroparesis including adverse events and limitations as well as future directions in pharmacologic research. INTRODUCTION astroparesis is a syndrome characterized by documented gastroparesis are increasing.2 Physicians delayed emptying of gastric contents without have both medical and surgical approaches for these Gmechanical obstruction of the stomach, pylorus or patients (See Figure 1). Medical therapy includes both small bowel. Patients can present with nausea, vomiting, prokinetics and antiemetics (See Table 1 and Table 2). postprandial fullness, early satiety, pressure, fullness The gastroparesis population will grow as diabetes and abdominal distension. In addition, abdominal pain increases and new therapies will be required. What located in the epigastrium, and distinguished from the do we know about the size of the gastroparetic term discomfort, is increasingly being recognized population? According to a study from the Mayo Clinic as an important symptom. The main etiologies of group surveying Olmsted County in Minnesota, the gastroparesis are diabetes, idiopathic, and post gastric risk of gastroparesis in Type 1 diabetes mellitus was and esophageal surgeries.1 Hospitalizations from significantly greater than for Type 2.