Incidence of Visceral Leishmaniasis in the Vaishali District of Bihar, India: Spatial Patterns and Role of Inland Water Bodies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Constituency-Wise Information on Inclusion and Deletions in Current Electoral Over Previous Roll
ELECTION COMMISSION OF INDIA Format 4B Format 4B (With CEO) Constituency-wise Information on Inclusion and Deletions in Current Electoral Over Previous Roll Name of State: BIHAR Net % Total claims lodged in Total Objections Lodged in Suo-moto Deletion Electors as per proposed Final change Change Electors as per Draft Roll w.r.t. Total Deletions subsequent Assembly Constituency Form 6 after draft Total Claims admitted Form 7 after draft publication Total Objections admitted subsenquent to last Number of Deletions Due to Roll w.r.t. 01.01.2021 as the over over 01.01.2021 as the qualifying date to last publication of roll publication of roll of roll pulication of roll qualifying date previous previuos Final roll Final roll Third Third Third Third Third Third Third Third No Name Male Female Male Female Male Female Male Female Male Female Male Female Male Female Expired Shifted Repeated Male Female (+/-) (+/-) Gender Gender Gender Gender Gender Gender Gender Gender 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 1 Valmiki Nagar 178264 153489 34 6317 5476 3 85 60 0 0 0 0 85 60 0 85 60 0 184499 158902 37 11651 3.39 2 Ramnagar (SC) 155977 139899 10 3372 2676 0 46 28 0 0 0 0 46 28 0 0 1 62 159305 142545 10 5974 1.98 3 Narkatiaganj 141813 123638 16 3340 2798 1 345 296 0 0 0 0 345 296 0 468 80 70 144815 126133 17 5498 2.03 4 Bagaha 162404 142895 15 4055 3721 1 142 155 0 0 0 0 142 155 0 147 25 121 166316 146462 16 7480 2.39 5 Lauriya 137451 118586 11 1999 1647 0 87 68 0 0 0 0 87 68 0 96 28 29 139363 120165 11 3491 -

Of India 100935 Parampara Foundation Hanumant Nagar ,Ward No
AO AO Name Address Block District Mobile Email Code Number 97634 Chandra Rekha Shivpuri Shiv Mandir Road Ward No 09 Araria Araria 9661056042 [email protected] Development Foundation Araria Araria 97500 Divya Dristi Bharat Divya Dristi Bharat Chitragupt Araria Araria 9304004533 [email protected] Nagar,Ward No-21,Near Subhash Stadium,Araria 854311 Bihar Araria 100340 Maxwell Computer Centre Hanumant Nagar, Ward No 15, Ashram Araria Araria 9934606071 [email protected] Road Araria 98667 National Harmony Work & Hanumant Nagar, Ward No.-15, Po+Ps- Araria Araria 9973299101 [email protected] Welfare Development Araria, Bihar Araria Organisation Of India 100935 Parampara Foundation Hanumant Nagar ,Ward No. 16,Near Araria Araria 7644088124 [email protected] Durga Mandir Araria 97613 Sarthak Foundation C/O - Taranand Mishra , Shivpuri Ward Araria Araria 8757872102 [email protected] No. 09 P.O + P.S - Araria Araria 98590 Vivekanand Institute Of 1st Floor Milan Market Infront Of Canara Araria Araria 9955312121 [email protected] Information Technology Bank Near Adb Chowk Bus Stand Road Araria Araria 100610 Ambedkar Seva Sansthan, Joyprakashnagar Wardno-7 Shivpuri Araria Araria 8863024705 [email protected] C/O-Krishnamaya Institute Joyprakash Nagar Ward No -7 Araria Of Higher Education 99468 Prerna Society Of Khajuri Bazar Araria Bharga Araria 7835050423 [email protected] Technical Education And ma Research 100101 Youth Forum Forbesganj Bharga Araria 7764868759 [email protected] -
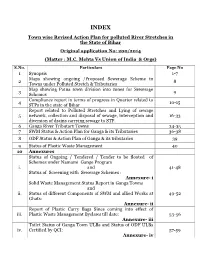
Town Wise Revised Action Plan for Polluted River Stretches in the State of Bihar Original Application No: 200/2014 (Matter : M.C
INDEX Town wise Revised Action Plan for polluted River Stretches in the State of Bihar Original application No: 200/2014 (Matter : M.C. Mehta Vs Union of India & Orgs) S.No. Particulars Page No 1 Synopsis 1-7 Maps showing ongoing /Proposed Sewerage Scheme in 2 8 Towns under Polluted Stretch & Tributaries Map showing Patna town division into zones for Sewerage 3 9 Schemes Compliance report in terms of progress in Quarter related to 4 10-15 STPs in the state of Bihar Report related to Polluted Stretches and Lying of sewage 5 network, collection and disposal of sewage, interception and 16-33 diversion of drains carrying sewage to STP. 6 Ganga River Tributary Towns 34-35 7 SWM Status & Action Plan for Ganga & its Tributaries 36-38 8 ODF Status & Action Plan of Ganga & its tributaries 39 9 Status of Plastic Waste Management 40 10 Annexures Status of Ongoing / Tendered / Tender to be floated of Schemes under Namami Gange Program i. and 41-48 Status of Screening with Sewerage Schemes : Annexure- i Solid Waste Management Status Report in Ganga Towns and ii. Status of different Components of SWM and allied Works at 49-52 Ghats: Annexure- ii Report of Plastic Carry Bags Since coming into effect of iii. Plastic Waste Management Byelaws till date: 53-56 Annexure- iii Toilet Status of Ganga Town ULBs and Status of ODF ULBs iv. Certified by QCI: 57-59 Annexure- iv 60-68 and 69 11 Status on Utilization of treated sewage (Column- 1) 12 Flood Plain regulation 69 (Column-2) 13 E Flow in river Ganga & tributaries 70 (Column-4) 14 Assessment of E Flow 70 (Column-5) 70 (Column- 3) 15 Adopting good irrigation practices to Conserve water and 71-76 16 Details of Inundated area along Ganga river with Maps 77-90 17 Rain water harvesting system in river Ganga & tributaries 91-96 18 Letter related to regulation of Ground water 97 Compliance report to the prohibit dumping of bio-medical 19 98-99 waste Securing compliance to ensuring that water quality at every 20 100 (Column- 5) point meets the standards. -

Master Plan for Patna - 2031
IMPROVING DRAFT MASTER PLAN FOR PATNA - 2031 FINAL REPORT Prepared for, Department of Urban Development & Housing, Govt. of Bihar Prepared by, CEPT, Ahmadabad FINAL REPORT IMPROVING DRAFT MASTER PLAN FOR PATNA-2031 FINAL REPORT IMPROVING DRAFT MASTER PLAN FOR PATNA - 2031 Client: Urban Development & Housing Department Patna, Bihar i Prepared by: Center for Environmental Planning and Technology (CEPT) University Kasturbhai Lalbhai Campus, University Road, Navrangpura, Ahmedabad – 380 009 Gujarat State Tel: +91 79 2630 2470 / 2740 l Fax: +91 79 2630 2075 www.cept.ac.in I www.spcept.ac.in CEPT UNIVERSITY I AHMEDABAD i FINAL REPORT IMPROVING DRAFT MASTER PLAN FOR PATNA-2031 TABLE OF CONTENTS TABLE OF CONTENTS i LIST OF TABLES v LIST OF FIGURES vii LIST OF MAPS viii LIST of ANNEXURE ix 1 INTRODUCTION 10 1.1 Introduction 11 1.2 Planning Significance of Patna as a City 12 1.3 Economic Profile 14 1.4 Existing Land Use – Patna Municipal Corporation Area 14 1.5 Previous Planning Initiatives 16 1.5.1 Master Plan (1961-81) 16 1.5.2 Plan Update (1981-2001) 17 1.5.3 Master Plan 2001-21 18 1.6 Need for the Revision of the Master Plan 19 1.7 Methodology 20 1.7.1 Stage 1: Project initiation 20 1.7.2 Stage 02 and 03: Analysis of existing situation & Future projections and Concept Plan 21 1.7.3 Stage 04: Updated Base Map and Existing Land Use Map 21 1.7.4 Stage 5: Pre-final Master Plan and DCR 24 2 DELINEATION OF PATNA PLANNING AREA 25 i 2.1 Extent of Patna Planning Area (Project Area) 26 2.2 Delineation of Patna Planning Area (Project Area) 27 2.3 Delineated -
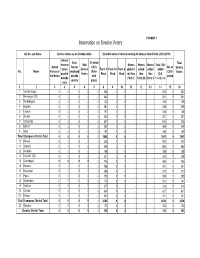
Eci Format (1-8)
FORMAT 7 Information on Service Voters AC No. and Name Service Voters as on De-Novo date Enrolled Service Voter According to Service Voter Portal (28.01.2019) Armed Govt. Of which Total force of Total Names Names Names Total SVs Armed Person CSVs No. of Service States Service Form 2 Form 2A Form 3 added in added added added No. Name Forces of employed (Voter CSVs Voters posted Voters Recd Recd Recd roll thru thru thru (Col. the Union outside with added outside Form 2 Form 2A Form 3 11+12+13) country proxy) state 1 2 3 4 5 6 7 8 9 10 11 1213 14 1516 1 Valmiki Nagar 0 0 0 0 0253 0 0 - - - 232 0 232 2 Ramnagar (SC) 0 0 0 0 0282 0 2 - - - 261 0 261 3 Narkatiaganj 0 0 0 0 0142 0 0 - - - 129 0 129 4 Bagaha 0 0 0 0 0281 0 1 - - - 259 0 259 5 Lauriya 0 0 0 0 0191 0 0 - - - 186 0 186 6 Nautan 0 0 0 0 0260 0 0 - - - 251 0 251 7 Chanpatia 0 0 0 0 0607 0 0 - - - 576 0 576 8 Bettiah 0 0 0 0 0467 0 2 - - - 459 0 459 9 Sikta 0 0 0 0 0197 0 0 - - - 189 0 189 West Champaran District Total 0 0 0 0 0 2680 0 5 --- 2542 0 2542 10 Raxaul 0 0 0 0 0309 0 0 - - - 290 0 290 11 Sugauli 0 0 0 0 0608 0 0 - - - 586 0 586 12 Narkatia 0 0 0 0 0198 0 0 - - - 188 0 188 13 Harsidhi (SC) 0 0 0 0 0321 0 4 - - - 305 0 305 14 Govindganj 0 0 0 0 0476 0 1 - - - 453 0 453 15Kesaria 0 0 0 0 0286 0 0 - - - 261 0 261 16Kalyanpur 0 0 0 0 0286 0 0 - - - 270 0 270 17Pipra 0 0 0 0 0279 0 0 - - - 259 0 259 18Madhuban 0 0 0 0 0173 0 0 - - - 161 0 161 19Motihari 0 0 0 0 0371 0 1 - - - 313 0 313 20 Chiraia 0 0 0 0 0235 0 2 - - - 221 0 221 21 Dhaka 0 0 0 0 0250 0 0 - - - 221 0 221 East Champaran District Total 0 0 0 0 0 3792 0 8 --- 3528 0 3528 22Sheohar 0 0 0 0 0172 0 2 - - - 153 0 153 Sheohar District Total 0 0 0 0 0 172 0 2 --- 153 0 153 AC No. -
Vaishali Introduction
DISTRICT PROFILE VAISHALI INTRODUCTION Vaishali district is one of the thirty-eight districts of the state of Bihar. It formed in 1972, separated from Muzaffarpur district. The district is surrounded by Muzaffarpur district in the North, Samastipur district in the East and Ganga River in South and Gandak River in West. The Vaishali district is a part of Tirhut division and the district headquarters are at Hajipur town. Hajipur is separated from the State’s biggest city Patna by a River Ganga. It is the second fastest growing city in the state. HISTORICAL BACKGROUND Vaishali got its name from King Vishal, a predecessor to Lord Ram. Vaishali finds reference in the Indian epics Ramayana. Vaishali was the capital of the Lichchavi State, considered as the First Republic in the World. It is said that the Lord Buddha, delivered his last semon and announced his Mahaparinirvana during his visit to Vaishali. 100 years after the Lord Buddha attained Mahaparinirvana, second Buddhist Council was held at Vaishali. Jain Tirthankar Lord Mahavir was said to be born at Vaishali to King Siddhartha and Queen Trishala. Amrapali the famous courtesan, has invited Lord Buddha to her house and Lord has visited her place. With Lord Buddha’s visit, Amrapali was purged with all impurities, she gifted her mango grove to the Sangh and joined Buddhism. Ananda, the favorite disciple of Buddha, attained Nirvana in the midst of Ganga outside Vaishali. ADMINISTRATIVE Hajipur City is the district headquarters. Vaishali district spread across 3 talukas: Mahnar, Hajipur, Mahua Vaishali district has been divided into 16 Municipal Blocks: o Mahnar o Hajipur o Chehrakala o Vaishali o Mahua o Premraj o Bidupur o Jandaha o Patedhi-Belshar o Goraul o Patepur o Desri o Raghopur o Sahadi buzurg o Lalganj o Bahgwanpur Total Number of Panchayats in Vaishali district 291. -

District Health Society Vaishali
District Health Plan 2009-2010 District Health Society Vaishali Table of contents Foreword About the Profile CHAPTER 1- INTRODUCTION 1.1 Background 1.2 Objectives of the process 1.3 Process of Plan Development 1.3.1 Preliminary Phase 1.3.2 Main Phase - Horizontal Integration of Vertical Programmes 1.3.3 Preparation of DHAP CHAPTER 2- DISTRICT PROFILE History Geographic Location Govt administrative setup Administrative units and towns. District Health Administrative setup Vaishali at a Glance Comparative Population Data 2.1 Socio economic Profile 2.2 Health Profile Indicators of Reproductive health and Child health 2.2.1 Health Status and Burden of diseases 2.2.2 Public Health Care delivery system 2.3 Map showing specialist doctors position 2.4 Map showing PHC and APHC locations 2.5 DLHS 3 data CHAPTER 3- SITUATION ANALYSIS 3.1 Gaps in infrastructure 3.1.1 HSC Infrastructure 3.1.2 Services of HSC 3.1.3 HSC Human Resource 3.2 APHC 3.3 PHC 3.4 District Hospital CHAPTER 4-Setting Objectives and suggested Plan of Action 4.1 Introduction 4.2 Targeted objectives and suggested Strategies 4.3 Maternal Health 4.4 Child Health 4.5 Family Planning 4.6 Kala-azar program 4.7 Blindness Control Program 4.8 Leprosy Eradication Program 4.9 Tuberculosis control Program 4.10 Filaria Control Prgram 4.11 Institution Strengthening 4.12 Program wise Budget 4.13 HIV/AIDS Foreword Recognizing the importance of Health in the process of economic and social development and improving the quality of life of our citizens, the Government of India has resolved to launch the National Rural Health Mission to carry out necessary architectural correction in the basic health care delivery system. -

Zoology Candidate ID Application No
Zoology Candidate ID Application No. Name Alloted College Admission Category 231821 516542833794 RANA AZAM A.P.J. Abdul Kalam Degree Mahavidyalaya, Pakdi Devraj, RamNagar, West Champaran General 143502 654194546994 RUKAIYA KHATOON A.P.J. Abdul Kalam Degree Mahavidyalaya, Pakdi Devraj, RamNagar, West Champaran General 32378 3357004136 NEHA KUMARI A.P.J. Abdul Kalam Degree Mahavidyalaya, Pakdi Devraj, RamNagar, West Champaran EBC 138647 887621193155 SHABBU KHATOON A.P.J. Abdul Kalam Degree Mahavidyalaya, Pakdi Devraj, RamNagar, West Champaran General 106234 491154131131 arun kumar A.P.J. Abdul Kalam Degree Mahavidyalaya, Pakdi Devraj, RamNagar, West Champaran General 141670 541902588848 DIVYANSHU KUMAR A.P.J. Abdul Kalam Degree Mahavidyalaya, Pakdi Devraj, RamNagar, West Champaran General 114918 174532912236 SHAMIMA KHATOON Akshaywat College, Mahua, Vaishali Dependent 135157 697941456638 PANKAJ KUMAR Akshaywat College, Mahua, Vaishali SC 196710 756038649981 MANISHA KUMARI Akshaywat College, Mahua, Vaishali NRI Quota 219679 884890308153 SEBY RAJ Akshaywat College, Mahua, Vaishali General 189628 039100359712 Md Ahmed Akshaywat College, Mahua, Vaishali General Backward Women 51202 938901595241 Divya Darpan Akshaywat College, Mahua, Vaishali (WBC) 74907 402576332926 MANISHA KUMARI Akshaywat College, Mahua, Vaishali BC 75153 418193532350 RISHABH RAJ Akshaywat College, Mahua, Vaishali General 77682 037645303244 ANJALI KUMARI Akshaywat College, Mahua, Vaishali EBC 88998 109820609415 VIKASH KUMAR Akshaywat College, Mahua, Vaishali SC 103323 600691894516 -

BIHAR District: PATNA
NATIONAL FEDERATION OF THE BLIND Plot No.-21,Sec-VI,M.B.Road,Pushp Vihar,New Delhi-110017 Membership List: BIHAR District: PATNA S.N. I-Card No. Name & Address Disability Date of Renewal M.Type Membership Date 1 BR-A-039 ABHAY RAM BLIND 05/12/2018 07/30/2020 ORDINARY S/O GORAKHNATH Age Renewal DOB ROAD NO-1 ASHOK NAGAR KANKABAGH Receipt No. PATNA BIHAR 800020 35 - - 69703 2 BR-A-054 ABHIJEET KUMAR SIGHTED 02/01/2019 07/30/2020 ORDINARY S/O KAMLA PRASAD GUPTA Age Renewal DOB NEAR BUS STAND SHANKARPUR BALI GANJ, IMAMGANJ PATNA, BIHAR Receipt No. 01-02-1985 69704 3 BR-A-069 ABHISEK KUMAR BLIND 02/01/2019 07/30/2020 ORDINARY S/O AJAY KUMAR THAKUR Age Renewal DOB GAYTRI NAGAR, DHIBRAPAR JEHANABAD BIHAR Receipt No. 08-10-1995 69705 4 BR-A-042 ADITIYA NARAYAN TIWARI BLIND 05/12/2018 07/30/2020 ORDINARY S/O SH. AMBIKA TIWARI Age Renewal DOB LAUKAHA, LAUKAHA MANKARARIYA EAST Receipt No. CHAMPARAN BIHAR 24 69706 5 BR-A-066 AFSARI KHATOON SIGHTED 02/01/2019 07/30/2020 ORDINARY D/O MOHAMMED ASHRAF Age Renewal DOB SULTANGANJ KHANMIRZA MASJID GHAT PATNA, MAHENDRU PATNA , BIHAR Receipt No. 05-07-1998 69707 6 BR-A-040 AFTAB ALAM (MOHD ) BLIND 05/12/2018 07/30/2020 ORDINARY S/O MD SOBRATI Age Renewal DOB NAUHATTA TAI DUMRI TAT DUMRI PATNA Receipt No. BIHAR 22YEARS 25/10/1996 69708 7 BR-A-073 AJAY KUMAR BLIND 02/01/2019 07/30/2020 ORDINARY S/O TEJALAL SAH Age Renewal DOB AT SISVANIYA WARD NO 01, PANCHYAT RAMPUR, PO. -

ANSWERED ON:01.12.2014 INCLUSION of CITES in WORLD HERITAGE Singh Shri Rama Kishore
GOVERNMENT OF INDIA CULTURE LOK SABHA UNSTARRED QUESTION NO:1327 ANSWERED ON:01.12.2014 INCLUSION OF CITES IN WORLD HERITAGE Singh Shri Rama Kishore Will the Minister of CULTURE be pleased to state: (a) whether the Government proposes to take steps for inclusion of various Indian cities in the UNESCO's World Heritage list; (b) if so, the details thereof and the present status of the cities along with the criteria adopted by the UNESCO to include ancient sites/ tourist places in World Heritage List; (c) whether the Government has identified or received any proposal from various State Governments to include places including Delhi and Vaishali in Bihar in the World Heritage list; (d) if so, the details thereof and the status of the proposals along with the time by which these places are likely to be considered by the World Heritage Committee, UNESCO for inclusion in the list; (e) whether the Government has conducted any survey to study the ancient historical importance of cities including Vaishali in Bihar; and (f) if so, the details thereof? Answer MINISTER OF STATE, CULTURE AND TOURISM (INDEPENDENT CHARGE) AND MINISTER OF STATE, CIVIL AVIATION (DR.MAHESH SHARMA) (a) Yes, Madam. (b) The nomination dossiers of Delhi Imperial Capital Cities and The Victorian & Art Deco Ensemble of Mumbai have been submitted to World Heritage Centre in January, 2014. The International Council on Monuments and Sites (ICOMOS) ICOMOS mission visited in October, 2014 to evaluate the property of Delhi Imperial Capital Cities. The decision to declare Delhi as World Heritage City would be taken in the World Heritage Committee meeting 2015. -

Social Changes Among the Scheduled Caste Population of the Vaishali District: a Geographical Study
IOSR Journal Of Humanities And Social Science (IOSR-JHSS) Volume 22, Issue 7, Ver.13 (July.2017) PP 34-41 e-ISSN: 2279-0837, p-ISSN: 2279-0845. www.iosrjournals.org Social Changes among the Scheduled Caste Population of the Vaishali District: A Geographical Study. * Sanjay Kumar Corresponding Author: Sanjay Kumar *UGC (NET) Qualified Research Scholar, College of Commerce, Arts & Science (Magadh University), Patna-20 Abstract: Change in social conditions concerns; transformation of culture, behaviour, social institutions and social structure of a society over time . It has taken place in most areas but as far as the less developed areas are concerned these have recorded phenomenal changes during the recent years due to improved educational facilities, economic conditions, mass- media communication, efforts of the social reformers, government policies, etc. So also the less developed areas of the State of Bihar have experienced significant social changes. The district of Vaishali, one of the country's 250 most backward districts, as by the ministry of Panchayati Raj identified in 2006, has also recorded considerable changes in the attitudinal, behavioural and structural features of the Scheduled Castes. The present paper aims to highlight the changes which have taken place among different Scheduled Castes of the selected villages of the Vaishali district. The paper highlights the changes in the social conditions of the migrant and non-migrant Scheduled Caste people with special reference to some of the social features like family structure, housing conditions, educational development, religious activities, dress pattern, changes in food habit & socialization pattern, etc. Keywords: Social change, Migration. Social change: Social change is an alteration in the Cultural, Structural, Population or Ecological characteristics of a social system. -

Information on Service Voters
FORMAT 7 Information on Service Voters Enrolled Service Voter According to Service AC No. and Name Enrolled Service Voter According to Service Voter Portal as on 08.02.2021 Voter Portal (28.12.2020) Armed Govt. Of which force of Total Names Names Names Total SVs Armed Person CSVs No. of Total States Service Form 2 Form 2A Form 3 added in added added added No. Name Forces of employed (Voter CSVs Service posted Voters Recd Recd Recd roll thru thru thru (Col. the Union outside with added Voters outside Form 2 Form 2A Form 3 11+12+13) country proxy) state 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 1 Valmiki Nagar - - - 292 0 1 0 0 1 0 0 1 - 293 2 Ramnagar (SC) - - - 321 0 0 0 0 0 0 0 0 - 321 3 Narkatiaganj - - - 165 0 0 0 0 0 0 0 0 - 165 4 Bagaha - - - 308 0 0 0 0 0 0 0 0 - 308 5 Lauriya - - - 229 0 0 0 0 0 0 0 0 - 229 6 Nautan - - - 313 0 0 0 0 0 0 0 0 - 313 7 Chanpatia - - - 679 0 2 0 0 2 0 0 2 - 681 8 Bettiah - - - 538 0 4 0 0 3 0 0 3 - 541 9 Sikta - - - 223 0 0 0 0 0 0 0 0 - 223 West Champaran District Total - - - 3068 0 7 0 0 6 0 0 6 - 3074 10 Raxaul - - - 359 0 0 0 0 0 0 0 0 - 359 11 Sugauli - - - 701 0 1 0 0 1 0 0 1 - 702 12 Narkatia - - - 243 0 0 0 0 0 0 0 0 - 243 13 Harsidhi (SC) - - - 389 0 0 0 0 0 0 0 0 - 389 14 Govindganj - - - 552 0 1 0 0 1 0 0 1 - 553 15 Kesaria - - - 339 0 0 0 0 0 0 0 0 - 339 16 Kalyanpur - - - 340 0 0 0 0 0 0 0 0 - 340 17 Pipra - - - 330 0 1 0 0 1 0 0 1 - 331 18 Madhuban - - - 195 0 1 0 0 0 0 0 0 - 195 19 Motihari - - - 407 0 0 0 0 0 0 0 0 - 407 20 Chiraia - - - 268 0 0 0 0 0 0 0 0 - 268 21 Dhaka - - - 269 0 0 0 0 0 0 0 0 - 269 East Champaran District Total - - - 4392 0 4 0 0 3 0 0 3 - 4395 22 Sheohar - - - 180 0 0 0 0 0 0 0 0 - 180 Sheohar District Total - - - 180 0 0 0 0 0 0 0 0 - 180 Enrolled Service Voter According to Service AC No.