RMTC Position Statement on Clenbuterol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Separation of Β-Receptor Blockers and Analogs by Capillary Liquid Chromatography
ORIGINAL ARTICLES College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, China Separation of b-receptor blockers and analogs by Capillary Liquid Chromatography (CLC) and Pressurized Capillary Electrochromatography (pCEC) using a vancomycin chiral stationary phase column Zhongyi Chen, Su Zeng, Tongwei Yao Received September 15, 2006, accepted October 28, 2006 Tongwei Jao, Department of Pharmaceutical Analysis and Drug Metabolism, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310031, China [email protected] Pharmazie 62: 585–592 (2007) doi: 10.1691/ph.2007.8.6194 Enantiomeric separation of chiral pharmaceuticals was carried out by means of in capillary liquid chroma- tography (CLC) and pressurized capillary electrochromatography (pCEC) using a vancomycin chiral sta- tionary phase (CSP). A 100 mm I.D. fused-silica capillary was packed with 5 mm diameter silica particles modified with vancomycin. Enantiomeric resolution of fifteen b-receptor blockers and analogs was stu- died by polar organic CLC mode and reversed-phase pCEC mode using mobile phases containing methanol-isopropanol-acetic acid-triethylamine and TEAA buffer-methanol, respectively. Several factors affecting chiral separation were investigated in both CLC and pCEC mode. Good enantiomeric resolution was achieved by CLC mode for propranolol, celiprolol, esmolol, bisoprolol, atenolol, metoprolol and car- teolol using methanol-isopropanol-acetic acid-triethylamine (70 : 30 : 0.05 : 0.05, v/v/v/v) as mobile phase and for clenbuterol, bambuterol, terbutaline, and salbutamol using methanol-isopropanol-acetic acid- triethylamine (50 : 50 : 0.05 : 005 or 50 : 50: 0.025 : 0.05, v/v/v/v) as mobile phase. The baseline was achieved by pCEC mode for the separation of esmolol, bisoprolol, atenolol, metoprolol, carteolol in the mobile phase containing MeOH-0.05%TEAA (pH 7.0) (90 : 10, v/v) (–10 kV), and that of propranolol and celiprolol in the mobile phase containing MeOH-0.025%TEAA (pH 7.0) (90 : 10, v/v)(–10 kV). -

Clenbuterol Human Effects the Effect of Clenbuterol in Humans Is Researched Through Examining the History and Regulations of the Drug
Clenbuterol Human Effects The effect of clenbuterol in humans is researched through examining the history and regulations of the drug. Specifically, Alberto Contador’s case is considered. Tag Words: Clenbuterol; drugs; Beta-2 Agonist; Effects; Thermogenic; Fat; Harmful; Authors: Jessie Yeh, Horace Lau, Danielle Lovisone with Julie M. Fagan, Ph.D. Summary (written by Danielle Lovisone) As a sympathomimetic and Beta-2 agonist, clenbuterol have several deleterious effects on the human body. The drug acts as a thermogenic stimulant, increasing lean muscle mass and respiratory efficiency while reducing fat. Cases on clenbuterol, including animal tests and human occurrences, support these unnatural and potentially harmful effects. With this, athletes and body builders have recently increased their use of the drug. Particularly, Alberto Contador has recently been targeted for having traces of clenbuterol in a urine drug test. Contador claims, instead of doping, this trace amount was unknowingly received from ingesting beef in Spain during the 2010 Tour de France. Although clenbuterol is banned in most areas of the world, this explanation seems plausible because the drug is poorly regulated by organizations such as the FDA. To examine Contador’s case further, our group compiled research on clenbuterol to ultimately hypothesize that Contador received this trace amount from contaminated beef. Our findings were submitted to the World Anti-Doping Agency as part of our Service Project. Video Link Class project 2010 fall: www.youtube.com/watch?v=ZTeJiBvbLhA The Issue: Clenbuterol The Effects of Clenbuterol on the Human Body By Jessie Yeh What is Clenbuterol? Clenbuterol is a chemical compound closely resembling the structure of an amine. -

2019 Year in Review: Aerosol Therapy
2019 Year in Review: Aerosol Therapy Ariel Berlinski Introduction COPD Newly Approved Drugs Asthma New Devices As-Needed Inhaled Corticosteroid/Long-Acting Bronchodilator Therapy Asthma Medication Report in Adolescents and Caregivers Cystic Fibrosis Hypertonic Saline in Cystic Fibrosis Infectivity of Cough Aerosols in Cystic Fibrosis Liposomal Amikacin for MAC Lung Disease Electronic Nicotine Delivery Systems E-Cigarette or Vaping Associated Lung Injury Secondhand Exposure Summary Relevant publications related to medicinal and toxic aerosols are discussed in this review. Treatment of COPD includes a combination of long-acting bronchodilators and long-acting muscarinic antagonists. A combination of aclidinium bromide and formoterol fumarate was approved in the United States. The combination was superior to its components alone, as well as tiotropium and a salmeterol-fluticasone combination. Increased risk of an asthma exacerba- tion was reported in children exposed to electronic nicotine delivery systems. A smart inhaler capable of recording inspiratory flow was approved in the United States. The use of as-needed budesonide-formoterol was reported to be superior to scheduled budesonide and as-needed ter- butaline for the treatment of adults with mild-to-moderate asthma. A survey among teens with asthma and their caregivers revealed a disagreement in the number of inhaled controller medi- cations the teen was taking. Treatment with inhaled hypertonic saline resulted in a decreased lung clearance index in infants and preschool children with cystic fibrosis. Surgical masks were well tolerated and significantly decreased the burden of aerosolized bacteria generated by coughing in adults with cystic fibrosis. Inhaled liposomal amikacin in addition to guideline- based therapy was reported to be superior to guideline-based therapy alone in achieving nega- tive sputum cultures in adult subjects with Mycobacterium avium complex pulmonary disease. -

Terbutaline Sulfate Injection, USP
Terbutaline Sulfate Injection, USP 1 mg per mL | NDC 70860-801-01 ATHENEX AccuraSEESM PACKAGING AND LABELING BIG, BOLD AND BRIGHT — TO HELP YOU SEE IT, SAY IT AND PICK IT RIGHT DIFFERENTIATION IN EVERY LABEL, DESIGNED TO HELP REDUCE MEDICATION ERRORS PLEASE SEE FULL PRESCRIBING INFORMATION, INCLUDING BOXED WARNING, FOR TERBUTALINE SULFATE INJECTION, USP, ENCLOSED. THE NEXT GENERATION OF PHARMACY INNOVATION To order, call 1-855-273-0154 or visit www.Athenexpharma.com Terbutaline Sulfate Injection, USP 1 mg NDC 70860-801-01 1 mg per mL DESCRIPTION Glass Vial CONCENTRATION 1 mg per mL CLOSURE 13 mm UNIT OF SALE 10 vials BAR CODED Yes CHOOSE AccuraSEESM FOR YOUR PHARMACY Our proprietary, differentiated and highly-visible label designs can assist pharmacists in accurate medication selection. With a unique AccuraSEE label design for every Athenex product, we’re helping your pharmacy to reduce the risk of medication errors. The idea is simple: “So what you see is exactly what you get.” Athenex, AccuraSEE and all label designs are copyright of Athenex. ©2019 Athenex. APD-0022-02-4/19 To order, call 1-855-273-0154 or visit www.Athenexpharma.com TERBUTALINE SULFATE Injection, USP • The use of beta-adrenergic agonist bronchodilators • Terbutaline sulfate should be used during nursing alone may not be adequate to control asthma in only if the potential benefit justifies the possible risk INDICATIONS AND USAGE many patients. Early consideration should be given to to the newborn. • Terbutaline sulfate injection is indicated for the adding anti-inflammatory agents, e.g., corticosteroids. prevention and reversal of bronchospasm in patients ADVERSE REACTIONS 12 years of age and older with asthma and reversible • Terbutaline sulfate should be used with caution • Common adverse reactions reported with terbutaline bronchospasm associated with bronchitis and emphysema. -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Skeletal and Cardiac Muscle Ergogenics and Side Effects Of
edicine s M & rt D o o p p i S n f g o S Douillard, J Sport Medic Doping Studie 2011, S1 l Journal of Sports Medicine & Doping t a u n d r i e u DOI: 10.4172/2161-0673.S1-001 s o J Studies ISSN: 2161-0673 Review Article Open Access Skeletal and Cardiac Muscle Ergogenics and Side Effects of Clenbuterol Treatment Aymeric Douillard* INRA, UMR866 Dynamique Musculaire et Métabolisme, Université Montpellier 1, F-34060 Montpellier, France Abstract Well known, well detected and still used by athletes, clenbuterol is one of the β2-agonists which has no authorization for therapeutic use, contrarily to salbutamol, salmeterol and formoterol in the 2012 World Anti-Doping Agency list. However, clenbuterol is still detected in athletes’ antidoping test samples. Its ability to induce muscle hypertrophy but also its strong lipolytic action and the absence of androgenic effects have made it a prized substances by athletes, specially females, without scruples whose performance requires significant muscle strength. Like the effects of clenbuterol on the heart, the effects of clenbuterol on skeletal muscle are dependent on the doses used and duration of the treatment. If there is a consensus concerning the clenbuterol action on the phenotypic conversion from slow to fast type fibers and on the hypertrophy, there is, to our knowledge, no consensus concerning the effects of clenbuterol on the slow type fibers and slow profile muscles. There is also no consensus concerning the clenbuterol effects on performance. We will shortly reviewing the known operating mode, side and benefits effects of short and long term β-agonists, and specially clenbuterol, treatment on mammals. -

Tocolytic Therapy a Meta-Analysis and Decision Analysis
Tocolytic Therapy A Meta-Analysis and Decision Analysis David M. Haas, MD, MS, Thomas F. Imperiale, MD, Page R. Kirkpatrick, Robert W. Klein, Terrell W. Zollinger, DrPH, and Alan M. Golichowski, MD, PhD OBJECTIVE: To determine the optimal first-line tocolytic ing prostaglandin inhibitors, only 80 would deliver within agent for treatment of premature labor. 48 hours, compared with 182 for the next-best treatment. METHODS: We performed a quantitative analysis of ran- CONCLUSION: Although all current tocolytic agents domized controlled trials of tocolysis, extracting data on were superior to no treatment at delaying delivery for maternal and neonatal outcomes, and pooling rates for both 48 hours and 7 days, prostaglandin inhibitors were each outcome across trials by treatment. Outcomes were superior to the other agents and may be considered the delay of delivery for 48 hours, 7 days, and until 37 weeks; optimal first-line agent before 32 weeks of gestation to adverse effects causing discontinuation of therapy; absence delay delivery. of respiratory distress syndrome; and neonatal survival. We (Obstet Gynecol 2009;113:585–94) used weighted proportions from a random-effects meta- analysis in a decision model to determine the optimal first-line tocolytic therapy. Sensitivity analysis was per- reterm birth, defined as any birth before the gesta- formed using the standard errors of the weighted propor- Ptional age of 37 weeks, is responsible for most of the 1–3 tions. neonatal morbidity and mortality in the United States and consumes 35% of all U.S. healthcare spending on RESULTS: Fifty-eight studies satisfied the inclusion crite- 4 ria. -

WO 2014/141069 Al 18 September 2014 (18.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/141069 Al 18 September 2014 (18.09.2014) P O P C T (51) International Patent Classification: Novartis Pharmaceuticals Corporation, 150 Industrial A61K 9/00 (2006.01) A61K 31/00 (2006.01) Road, San Carlos, California 94070 (US). A61K 9/16 (2006.01) (74) Agent: MAZZA, Michael; Novartis Vaccines and Dia (21) International Application Number: gnostics, Inc., 4560 Horton Street, Emeryville, California PCT/IB2014/059632 94608 (US). (22) International Filing Date: (81) Designated States (unless otherwise indicated, for every 11 March 2014 ( 11.03.2014) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (25) Filing Language: English BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (26) Publication Language: English DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (30) Priority Data: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 61/784,865 14 March 2013 (14.03.2013) US MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (71) Applicant (for all designated States except US) : NO¬ OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, VARTIS AG [CH/CH]; Lichtstrasse 35, CH-4056 Basel SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (CH). -

2-Adrenergic Receptor Is Determined by Conformational Equilibrium in the Transmembrane Region
ARTICLE Received 21 Mar 2012 | Accepted 2 Aug 2012 | Published 4 Sep 2012 DOI: 10.1038/ncomms2046 Efficacy of theβ 2-adrenergic receptor is determined by conformational equilibrium in the transmembrane region Yutaka Kofuku1,2, Takumi Ueda1, Junya Okude1, Yutaro Shiraishi1, Keita Kondo1, Masahiro Maeda3, Hideki Tsujishita3 & Ichio Shimada1,4 Many drugs that target G-protein-coupled receptors (GPCRs) induce or inhibit their signal transduction with different strengths, which affect their therapeutic properties. However, the mechanism underlying the differences in the signalling levels is still not clear, although several structures of GPCRs complexed with ligands determined by X-ray crystallography are available. Here we utilized NMR to monitor the signals from the methionine residue at position 82 in neutral antagonist- and partial agonist-bound states of β2-adrenergic receptor (β2AR), which are correlated with the conformational changes of the transmembrane regions upon activation. We show that this residue exists in a conformational equilibrium between the inverse agonist- bound states and the full agonist-bound state, and the population of the latter reflects the signal transduction level in each ligand-bound state. These findings provide insights into the multi-level signalling of β2AR and other GPCRs, including the basal activity, and the mechanism of signal transduction mediated by GPCRs. 1 Graduate School of Pharmaceutical Sciences, The University of Tokyo, Hongo 7-3-1, Bunkyo-ku, Tokyo 113-0033, Japan. 2 Japan Biological Informatics Consortium (JBIC), Tokyo 135-0064, Japan. 3 Shionogi Co., Ltd., Discovery Research Laboratories, Osaka 561-0825, Japan. 4 Biomedicinal Information Research Center (BIRC), National Institute of Advanced Industrial Science and Technology (AIST), Aomi 2-41-6, Koto-ku, Tokyo 135-0064, Japan. -

210428Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 210428Orig1s000 OTHER REVIEW(S) DIVISION OF CARDIOVASCULAR AND RENAL PRODUCTS Regulatory Project Manager Overview I. GENERAL INFORMATION NDA: 210428 Drug: Metoprolol Succinate Extended-Release Capsules, 25 mg, 50 mg, 100 mg and 200 mg Class: Beta- Blocker Applicant: Sun Pharmaceutical Industries Limited Proposed Indications: Hypertension, Angina Pectoris & Heart Failure Date of submission: March 30, 2017 PDUFA date: January 30, 2018 II. REVIEW TEAM Office of New Drugs, Office of Drug Evaluation I: Division of Cardiovascular & Renal Product Norman Stockbridge, MD, PhD, Director Mary Ross Southworth, PharmD, Deputy Director for Safety Michael Monteleone, MS, RAC, Assistant Director for Labeling Martina Sahre, PhD, Cross Discipline Team Leader Fortunato Senatore, MD, Clinical Reviewer Albert DeFelice, PhD, Non-Clinical Supervisor Muriel Saulnier, PhD, Non-Clinical Reviewer Edward Fromm, RPh, RAC, Chief, Project Management Staff Maryam Changi, PharmD, Regulatory Project Manager Office of Pharmaceutical Quality: Wendy Wilson-Lee, PhD, Application Technical Lead Grafton Adams, Regulatory Business Process Manager Milton Sloan, PhD, Drug Product Reviewer Rohit Tiwari, PhD, Drug Substance Reviewer Ben Stevens, PhD, Drug Substance Team Leader Kaushal Dave, PhD, Biopharmaceutical Reviewer Wu, Ta-Chin, PhD, Biopharmaceutical Team Leader Viviana Matta, PhD, Facility Reviewer Ruth Moore, PhD, Facility Team Leader Chunsheng Cai, PhD, Process Reviewer NDA 210428 RPM review Page 1 Reference ID: 42132034213643 Labeling/PMC-PMR to Sponsor: November 30, 2017 PDUFA Date: January 30, 2018 (Standard, 10-Month) PDUFA Goal Date: January 30, 2018 Approval letter: January 26, 2018 7. Reviews a) Divisional Memorandum: (January 26, 2018) Dr. Stockbridge indicated his concurrence on Dr. Sahre’s CDTL memo. -

PRAC Draft Agenda of Meeting 11-14 May 2020
11 May 2020 EMA/PRAC/257460/2020 Human Division Pharmacovigilance Risk Assessment Committee (PRAC) Draft agenda for the meeting on 11-14 May 2020 Chair: Sabine Straus – Vice-Chair: Martin Huber 11 May 2020, 10:30 – 19:30, via teleconference 12 May 2020, 08:30 – 19:30, via teleconference 13 May 2020, 08:30 – 19:30, via teleconference 14 May 2020, 08:30 – 16:00, via teleconference Organisational, regulatory and methodological matters (ORGAM) 28 May 2020, 09:00-12:00, via teleconference Disclaimers Some of the information contained in this agenda is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also change during the course of the review. Additional details on some of these procedures will be published in the PRAC meeting highlights once the procedures are finalised. Of note, this agenda is a working document primarily designed for PRAC members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the agenda cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on-going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006, Rev. 1). Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. -
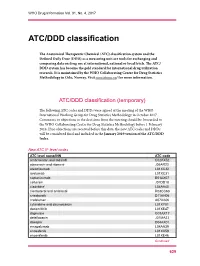
ATC/DDD Classification
WHO Drug Information Vol. 31, No. 4, 2017 ATC/DDD classification The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measuring unit are tools for exchanging and comparing data on drug use at international, national or local levels. The ATC/ DDD system has become the gold standard for international drug utilization research. It is maintained by the WHO Collaborating Centre for Drug Statistics Methodology in Oslo, Norway. Visit www.whocc.no/ for more information. ATC/DDD classification (temporary) The following ATC codes and DDDs were agreed at the meeting of the WHO International Working Group for Drug Statistics Methodology in October 2017. Comments or objections to the decisions from the meeting should be forwarded to the WHO Collaborating Centre for Drug Statistics Methodology before 1 February 2018. If no objections are received before this date, the new ATC codes and DDDs will be considered final and included in the January 2019 version of the ATC/DDD Index. New ATC 5th level codes ATC level name/INN ATC code ambrisentan and tadalafil C02KX52 atazanavir and ritonavir J05AR23 atezolizumab L01XC32 avelumab L01XC31 caplacizumab B01AX07 cefteram J01DD18 cladribine1 L04AA40 clenbuterol and ambroxol R03CC63 crisaborole D11AH06 crofelemer A07XA06 cytarabine and daunorubicin L01XY01 dacomitinib L01XE47 dapivirine G01AX17 delafloxacin J01MA23 doxepin D04AX01 emapalumab L04AA39 enasidenib L01XX59 encorafenib L01XE46 Continued 629 ATC/DDD classification WHO Drug Information Vol. 31, No. 4, 2017