Dikewater Relationships to Potential Geothermal Resources on Leeward West Maui, State of Hawaii
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RECORDS of the HAWAII BIOLOGICAL SURVEY for 1995 Part 2: Notes1
RECORDS OF THE HAWAII BIOLOGICAL SURVEY FOR 1995 Part 2: Notes1 This is the second of two parts to the Records of the Hawaii Biological Survey for 1995 and contains the notes on Hawaiian species of plants and animals including new state and island records, range extensions, and other information. Larger, more compre- hensive treatments and papers describing new taxa are treated in the first part of this Records [Bishop Museum Occasional Papers 45]. New Hawaiian Pest Plant Records for 1995 PATRICK CONANT (Hawaii Dept. of Agriculture, Plant Pest Control Branch, 1428 S King St, Honolulu, HI 96814) Fabaceae Ulex europaeus L. New island record On 6 October 1995, Hawaii Department of Land and Natural Resources, Division of Forestry and Wildlife employee C. Joao submitted an unusual plant he found while work- ing in the Molokai Forest Reserve. The plant was identified as U. europaeus and con- firmed by a Hawaii Department of Agriculture (HDOA) nox-A survey of the site on 9 October revealed an infestation of ca. 19 m2 at about 457 m elevation in the Kamiloa Distr., ca. 6.2 km above Kamehameha Highway. Distribution in Wagner et al. (1990, Manual of the flowering plants of Hawai‘i, p. 716) listed as Maui and Hawaii. Material examined: MOLOKAI: Molokai Forest Reserve, 4 Dec 1995, Guy Nagai s.n. (BISH). Melastomataceae Miconia calvescens DC. New island record, range extensions On 11 October, a student submitted a leaf specimen from the Wailua Houselots area on Kauai to PPC technician A. Bell, who had the specimen confirmed by David Lorence of the National Tropical Botanical Garden as being M. -
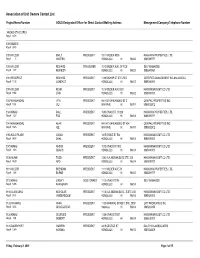
Association of Unit Owners Contact List
Association of Unit Owners Contact List Project Name/Number AOUO Designated Officer for Direct Contact/Mailing Address Management Company/Telephone Number `AKOKO AT HO`OPILI Reg.# 8073 1001 QUEEN Reg.# 7675 1001 WILDER EMILY PRESIDENT 1001 WILDER #305 HAWAIIAN PROPERTIES, LTD. Reg.# 5 WATERS HONOLULU HI 96822 8085399777 1010 WILDER RICHARD TREASURER 1010 WILDER AVE, OFFICE SELF MANAGED Reg.# 377 KENNEDY HONOLULU HI 96822 8085241961 1011 PROSPECT RICHARD PRESIDENT 1188 BISHOP ST STE 2503 CERTIFIED MANAGEMENT INC dba ASSOCI Reg.# 1130 CONRADT HONOLULU HI 96813 8088360911 1015 WILDER KEVIN PRESIDENT 1015 WILDER AVE #201 HAWAIIANA MGMT CO LTD Reg.# 1960 LIMA HONOLULU HI 96822 8085939100 1037 KAHUAMOKU VITA PRESIDENT 94-1037 KAHUAMOKU ST 3 CEN PAC PROPERTIES INC Reg.# 1551 VILI WAIPAHU HI 96797 8085932902 1040 KINAU PAUL PRESIDENT 1040 KINAU ST., #1206 HAWAIIAN PROPERTIES, LTD. Reg.# 527 FOX HONOLULU HI 96814 8085399777 1041 KAHUAMOKU ALAN PRESIDENT 94-1041 KAHUAMOKU ST 404 CEN PAC PROPERTIES INC Reg.# 1623 IGE WAIPAHU HI 96797 8085932902 1054 KALO PLACE JUANA PRESIDENT 1415 S KING ST 504 HAWAIIANA MGMT CO LTD Reg.# 5450 DAHL HONOLULU HI 96814 8085939100 1073 KINAU ANSON PRESIDENT 1073 KINAU ST 1003 HAWAIIANA MGMT CO LTD Reg.# 616 QUACH HONOLULU HI 96814 8085939100 1108 AUAHI TODD PRESIDENT 1240 ALA MOANA BLVD STE. 200 HAWAIIANA MGMT CO LTD Reg.# 7429 APO HONOLULU HI 96814 8085939100 1111 WILDER BRENDAN PRESIDENT 1111 WILDER AVE 7A HAWAIIAN PROPERTIES, LTD. Reg.# 228 BURNS HONOLULU HI 96822 8085399777 1112 KINAU LINDA Y SOLE OWNER 1112 KINAU ST PH SELF MANAGED Reg.# 1295 NAKAGAWA HONOLULU HI 96814 1118 ALA MOANA NICHOLAS PRESIDENT 1118 ALA MOANA BLVD., SUITE 200 HAWAIIANA MGMT CO LTD Reg.# 7431 VANDERBOOM HONOLULU HI 96814 8085939100 1133 WAIMANU ANNA PRESIDENT 1133 WAIMANU STREET, STE. -

Topographic History of the Maui Nui Complex, Hawai'i, and Its Implications for Biogeography1
Topographic History ofthe Maui Nui Complex, Hawai'i, and Its Implications for Biogeography 1 Jonathan Paul Price 2,4 and Deborah Elliott-Fisk3 Abstract: The Maui Nui complex of the Hawaiian Islands consists of the islands of Maui, Moloka'i, Lana'i, and Kaho'olawe, which were connected as a single landmass in the past. Aspects of volcanic landform construction, island subsi dence, and erosion were modeled to reconstruct the physical history of this complex. This model estimates the timing, duration, and topographic attributes of different island configurations by accounting for volcano growth and subsi dence, changes in sea level, and geomorphological processes. The model indi cates that Maui Nui was a single landmass that reached its maximum areal extent around 1.2 Ma, when it was larger than the current island of Hawai'i. As subsi dence ensued, the island divided during high sea stands of interglacial periods starting around 0.6 Ma; however during lower sea stands of glacial periods, islands reunited. The net effect is that the Maui Nui complex was a single large landmass for more than 75% of its history and included a high proportion of lowland area compared with the contemporary landscape. Because the Hawaiian Archipelago is an isolated system where most of the biota is a result of in situ evolution, landscape history is an important detertninant of biogeographic pat terns. Maui Nui's historical landscape contrasts sharply with the current land scape but is equally relevant to biogeographical analyses. THE HAWAIIAN ISLANDS present an ideal logic histories that can be reconstructed more setting in which to weigh the relative influ easily and accurately than in most regions. -

U-Series Isotopic Constraints on Mantle Upwelling on the Periphery of the Hawaiian Plume Isotope Geochemistry of Haleakala Crater Basanites Erin H
U-series isotopic constraints on mantle upwelling on the periphery of the Hawaiian plume Isotope geochemistry of Haleakala crater basanites Erin H. Phillips and Kenneth W.W. Sims, University of Wyoming, and Vincent J.M. Salters, Florida State University 1. Introduction 2. Major and Trace Element Geochemistry and Long-lived Radiogenic Isotopes 100 400 ● The Hawaiian islands are part of the age progressive series of 16 Comparison to Haleakala Crater (this study) 90 global alkaline volcanoes that form the Hawaiian-Emperor seamount chain. At 14 Haleakala SW Rift Zone (Sims et al., 1999) 300 suites Haleakala, on the island of Maui, basanitic lavas of the Hana Haleakala post-shield 80 12 Haleakala shield 200 Volcanics represent end-member rejuvenated stage alkaline 70 Kilauea magmatism (Clauge, 1987). Although shield-stage tholeiitic 100 10 Mauna Loa 60 volcanism predominates in the Hawaiian islands, alkaline lavas O 2 Mauna Kea 0 erupted on the trailing edge of the Hawaiian plume present an 8 Hualalai trachytes 50 O+Na important component to understanding mantle melting and solid 2 Loihi K 40 6 Nyiragongo mantle upwelling. Averages for 30 Leucite Hills lamproites Hawaiian Lavas 4 Ross Island, Antarctica basanites 20 ● We present geochemical data for 13 samples from Haleakala Samoa crater. 14C ages for seven samples range from 870 ± 40 to 4070 ± 50 2 See supplement for full reference list 10 years (Sherrod and McGeehin, 1999). Preliminary data for 5 0 1995) Mantle (McDonough and Sun, Sample/Primitive 0 samples from the Haleakala southwest rift zone (Sims et al., 1999) 35 40 45 50 55 60 65 Rb Ba Th U Nb La Ce Sr Pb Nd Sm Hf Eu Dy Y Yb Lu SiO are consistent with iso-viscous (Watson and McKenzie, 1991) and 2 thermo-viscous (Hauri et al., 1994) fluid mechanical models of 10 plume upwelling in which upwelling rates are slower on the Hawaiian Lavas periphery of plume. -

A Prodromus of the Water Bear Fauna of Haleakala National Park
A PRODROMUS OF THE WATER BEAR FAUNA OF HALEAKALA NATIONAL PARK Samuel M. Gon III Department of Entomology University of Hawai'i Honolulu, Hawai'i 96822 Robert A. Kimsey and Robert O. Schuster Department of Entomology University of California Davis, California 95616 Mark A. Willis Department of Entomology University of California Riverside, California 92507 ABSTRACT: The terrestrial meiofauna of the Hawaiian islands is almost completely unknown to science. Water bears (Tardigrada) represent a phylum that seems preadapted for long distance dispersal to the Hawaiian islands, and here we present, for the first time, 31 species in 10 genera and four families. The diversity of species in an area smaller than 50 square miles means that the Haleakala site is the most tardigrade rich location yet found on the planet. These species occupy a highly specialized niche in the Central Crater District of Haleakala National Park that involves alpine and subalpine cryptogams subject to extremes of temperature and desiccation. Several of the Hawaiian species are capable of anhydrobiosis, which is probably the mechanism that allows their occupation of alpine habitats. We present arguments that suggest that the richest habitats for Hawaiian water bears will be in subalpine and alpine settings of Maui and Hawai'i. 15 INTRODUCTION The biota of the Hawaiian islands attracts biologists because of its diversity. We have all came to expect new and exciting things here; new species being among the more engaging discoveries. We report here a first glimpse of a fascinating phylum that has until now passed generally unnoticed in Hawaiian biology. The phylum is Tardigrada, comprised of small (50 to 1200 micron) creatures usually called water bears because of their appearance and deliberate "pawing" form of locomotion. -

Volcanic and Seismic Hazards on the Island of Hawaii
U.S. Department of the Interior / U.S. Geological Survey Volcanic and Seismic Hazards on the Island of Hawaii Volcanic and Seismic Hazards on the Island of Hawaii Lava flows entered Kalapana Gardens in December 1986. Front Cover: View of Kapoho village during the 1960 eruption before it was entirely destroyed. (Photographer unknown) Inside Front Cover (Photograph by J.D. Griggs) For sale by the U.S. Government Printing Office Superintendent of Documents, Mail Stop: SSOP, Washington, DC 20402-9328 ISBN 0-16-038200-9 Preface he eruptions of volcanoes often have direct, dramatic effects on the lives of people and Ton their property. People who live on or near active volcanoes can benefit greatly from clear, scientific information about the volcanic and seismic hazards of the area. This booklet provides such information for the residents of Hawaii so they may effectively deal with the special geologic hazards of the island. Identifying and evaluating possible geologic hazards is one of the principal roles of the U.S. Geological Survey (USGS) and its Hawaiian Volcano Observatory. When USGS scientists recognize a potential hazard, such as an impend ing eruption, they notify the appropriate govern ment officials, who in turn are responsible for advising the public to evacuate certain areas or to take other actions to insure their safety. This booklet was prepared in cooperation with the Hawaii County Civil Defense Agency. Volcanic and Seismic Hazards: Interagency Responsibilities Hawaii County National Park } Civil Defense C Service ./ Short-term hazard evaluation for the agencies responsible for public safety. Information on volcanic U.S. -

Downloaded 10/07/21 11:20 PM UTC JULY 2010 C a R L I S E T a L
2706 MONTHLY WEATHER REVIEW VOLUME 138 Numerical Simulations of Island-Scale Airflow over Maui and the Maui Vortex under Summer Trade Wind Conditions DANA L. CARLIS* Howard University Program in Atmospheric Sciences, Washington, D.C., and Honolulu Weather Forecast Office, National Weather Service, Honolulu, Hawaii YI-LENG CHEN Department of Meteorology, University of Hawaii at Manoa, Honolulu, Hawaii VERNON R. MORRIS Howard University Program in Atmospheric Sciences, Howard University, Washington, D.C. (Manuscript received 22 September 2009, in final form 14 January 2010) ABSTRACT The fifth-generation Pennsylvania State University–NCAR Mesoscale Model (MM5) coupled with the Noah land surface model (LSM) is employed to simulate island-scale airflow and circulations over Maui County, Hawaii, under summer trade wind conditions, during July–August 2005. The model forecasts are validated by surface observations with good agreement. In this study, it is shown that a previously known closed circulation over the Central Valley of Maui, or the Maui vortex, represents the northern cyclonic vortex of the dual-counter-rotating vortices in the lee of Haleakala, which extend up to the base of the trade wind inversion with a westerly reversed flow (.2ms21). At low levels, the northern cyclonic vortex is more pronounced than the southern anticyclonic vortex. The asymmetric structure of the dual vortices is related to the shape of Haleakala and the flow deflection by the West Maui Mountains. The Maui vortex has a relatively narrow east–west extent in the lowest levels, espe- cially at night, due to the deflected strong northerly/northeasterly winds from the windward foothills of the West Maui Mountains. -

San Diego Style Weddings Oct/Nov'11
honeymoon & travel Honeymoon destination HAWAII turn the page for two of San Diego Style Weddings’ favorite hawaiian honeymoon destinations: Kohala Coast on the Big Island and Po’ipu, Kauai SanDiegoStyleWeddingS.com | 335 paradise foundKohala Coast on Hawaii, the Big Island PICture thIS: the perfect destination engagement, wedding, honeymoon and vow renewal location all rolled into one perfect coast on one perfect island, the Kohala Coast on hawaii, the Big Island. here’s San Diego Style Weddings’ “BeSt oF” list of places to stay and things to do while on your trip. WRITTen By Holly Lafferty Best Spa experience proposing to their potential fiancées. The Lomilomi massage at The Fairmont Consider this story shared by Jaisy Jar- Orchid, Hawaii’s Spa Without Walls is a dine, Director of Public Relations: “Re- unique experience in that the technique cently, a couple was basking in the after- is Hawaiian and while, like most mas- glow of an oceanfront couples massage sages, you won’t want it to end, you’ll in one of our ocean side cabanas when a know sadly that this dreamy version canoe carrying one of our orchid Beach is not available back on the mainland. Boys arrived along the shore bearing not only that, the individual or couple’s chilled champagne, chocolate covered massages are offered by a waterfall or strawberries, and a “Puolo” (a tradition- oceanside—neither view is too shabby. al gift bundle) wrapped in ti-leaves. The Request Tami and you’re sure to get the soon-to-be bride had no idea that inside best The Fairmont Orchid, Hawaii has the Puolo was a diamond ring. -

GRAND WAILEA FACT SHEET Situated on 40 Acres Fronting
GRAND WAILEA FACT SHEET Situated on 40 acres fronting spectacular Wailea Beach on the southwest shore of the island of Maui, the Grand Wailea is a 35-minute drive from the Kahului Airport and a 20-minute flight from Honolulu. Grand Wailea was built in 1991 with a commitment to portray the richness of Hawaii’s nature, culture and people. The resort features luxurious accommodations, award- winning cuisine, the unparalleled Spa Grande, championship golf and tennis, as well as the island’s most notable pools and water park. ACCOMMODATIONS The resort has 776 guestrooms, including 56 suites. The Napua Tower offers 100 rooms in a private club-area situated at the center of the hotel, featuring dedicated concierge services with upgraded guest room accommodations. Grand Wailea’s guest rooms are well designed and spacious, ranging from 640 square feet to 5,500 square feet. HO‛OLEI AT GRAND WAILEA The resort also offers luxury vacation villas with Ho‛olei at Grand Wailea - lavish private residences offering 3-bedroom, 3.5-bath luxury villas with the highest level of quality standards. Perfect for extended stays, special celebrations and family gatherings, Ho‛olei at Grand Wailea blends the finest in residential living, exceptional amenities and attentive services. Nestled in the center of the world-class Wailea Resort community and adjacent to Grand Wailea, the centerpiece of Ho‛olei at Grand Wailea is a lush tropical playground featuring a large pool with swim-in grotto and 19-foot waterfall, children's pool with a sandy beach entry and whirlpool tubs. Guests can enjoy a state-of-the-art fitness center with a private locker room complete with oversized showers and sauna. -

Island Studies Journal, Vol. 4, No. 2, 2009, Pp. 183-202 at The
Island Studies Journal , Vol. 4, No. 2, 2009, pp. 183-202 At the Intersection of Resident, Research and Recreation Stakeholder Interests: East Maui, Hawai‘i, as a Sustainable Tourism Destination John Cusick University of Hawai‘i at Manoa Oahu, Hawai‘i, USA [email protected] Abstract: The experiences of contemporary protected areas indicate adaptations to challenges brought about by resource management strategies. Resident communities, protected area management, and the tourism industry stakeholders demonstrate that evolving relationships are complex webs of competing and cooperating interests. The geographic isolation of East Maui delayed the cultural disruption of traditional practices and is an area where residents simultaneously resist assimilation and re-create cultural landscapes to offer visitors a glimpse into the past and a view of an emerging future associated with the renaissance of Native Hawaiian identity. Partnerships have brought about and nurtured the perpetuation of culture and the conservation of biodiversity as stakeholders recognize shared benefits. Among the outcomes are that residents have reconstituted the identity of East Maui as a Hawaiian place with benefits to various stakeholders, including a network of protected areas. A sustainability framework suggests a reappraisal of how to nurture, not alter, East Maui’s identity. Keywords : community based tourism, cultural kipuka, golf course development, Hana, Hawai‘i, islandness, Maui, protected areas, stakeholders, sustainability framework © 2009 – Institute of Island Studies, University of Prince Edward Island, Canada. Introduction The combination of biological diversity and cultural heritage is routinely cited as being conducive to the development of ecotourism activities (Honey, 1999; Stronza & Durham, 2008). An increase and expansion of tourism development associated with protected areas facilitate the convergence of stakeholder interests and raise issues related to resident place, research field site, and tourist destination (Howe et al ., 1997; Terborgh et al ., 2002). -

West Honolulu Watershed Study
West Honolulu Watershed Study Final Report Prepared For: Honolulu Board of Water Supply Department of Land and Natural Resources, Engineering Division U.S. Army Corps of Engineers, Honolulu District Prepared By: Townscape, Inc. and Eugene P. Dashiell, AICP May 2003 West Honolulu Watershed Study - Final Report - Prepared for: HONOLULU BOARD OF WATER SUPPLY DEPARTMENT OF LAND AND NATURAL RESOURCES ENGINEERING DIVISION U.S. ARMY CORPS OF ENGINEERS HONOLULU DISTRICT Prepared by: Townscape, Inc. and Eugene P. Dashiell, AICP May 2003 This page intentionally left blank. West Honolulu Watershed Study FINAL REPORT WEST HONOLULU WATERSHED STUDY ACKOWLEDGEMENTS This study was conducted under the direction of the U.S. Army Corps of Engineers, Honolulu District (COE), through Section 22 of the Water Resources Development Act of 1974, as amended. Project manager for the West Honolulu Watershed Study was Derek Chow of COE, Engineer District, Honolulu. Local sponsorship for the study was provided jointly by the City and County of Honolulu Board of Water Supply, represented by Barry Usagawa, Principal Executive of the Water Resources Unit, and Scot Muraoka, Long-Range Planning Section; and the State of Hawaiÿi Department of Land and Natural Resources (DLNR), Engineering Division, which was represented by Sterling Yong, Head of the Flood Control and Dam Safety Section, and Eric Yuasa and Carty Chang of the Project Planning Section. The three co-sponsors of this study wish to acknowledge the following groups and individuals for their contribution to the West Honolulu Watershed Study: Principal Planner and President Bruce Tsuchida and Staff Planners Michael Donoho and Sherri Hiraoka of Townscape, Inc., contracted through the COE as the planning consultant for the study. -

Platinum Hotels by Elaine Glusac Page: 1 2 3 4 5 6 7
Past Issues » Digital Editions » Videos THE LUXURY MAGAZINE FOR AMERICAN AIRLINES AND AMERICAN EAGLE PREMIUM CLASS PASSENGERS Home Travel Lifestyle Health & Fitness Food & Wine Business Platinum Hotels by Elaine Glusac Page: 1 2 3 4 5 6 7 Bellagio “The new luxury customer,” said John Vanderslice, global head of luxury and lifestyle brands including Waldorf for Hilton Worldwide, “wants an up-to-date service experience and authenticity.” In Hawaii, top pick The Ritz-Carlton, Kapalua nurtures island authenticity with environmental summer camps for kids, “walk ’n’ talk” history tours, and outrigger canoeing trips. But the resort also updated amenities to include a new adult pool, “dive-in” outdoor movies, and free dining at two restaurants for kids under the age of 12 with every paying adult. Down shore, the Four Seasons Resort Maui at wailea checks in stylishly with new pool cabanas designed by Missoni Home and a series of exclusive events including a November golf weekend with Philadelphia Phillies All-Star (and Maui native) Shane Victorino. Its neighbor Grand Wailea opened Amasia from chef Alan Wong in May, and recently began bottling the honey it harvests from hives on the ballroom roof. According to Matt Bailey, the resort’s managing director, the resort continually Top 5 U.S. Casino Resorts updates the majority of its offerings to generate buzz, whether that’s changing 1. Bellagio, Las Vegas the art gallery windows weekly or using (888) 987-6667, bellagio.com locally sourced salts in the spa baths. “People are looking for a more organic 2. Wynn Las Vegas experience,” he said.