Evaluating the Effects of Artificial Oxygenation and Hypoxia on Biota in the Upper Swan Estuary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Swan and Helena Rivers Management Framework Heritage Audit and Statement of Significance • FINAL REPORT • 26 February 2009
Swan and Helena Rivers Management Framework Heritage Audit and Statement of Significance • FINAL REPORT • 26 FEbRuARy 2009 REPORT CONTRIBUTORS: Alan Briggs Robin Chinnery Laura Colman Dr David Dolan Dr Sue Graham-Taylor A COLLABORATIVE PROJECT BY: Jenni Howlett Cheryl-Anne McCann LATITUDE CREATIVE SERVICES Brooke Mandy HERITAGE AND CONSERVATION PROFESSIONALS Gina Pickering (Project Manager) NATIONAL TRUST (WA) Rosemary Rosario Alison Storey Prepared FOR ThE EAsTERN Metropolitan REgIONAL COuNCIL ON bEhALF OF Dr Richard Walley OAM Cover image: View upstream, near Barker’s Bridge. Acknowledgements The consultants acknowledge the assistance received from the Councillors, staff and residents of the Town of Bassendean, Cities of Bayswater, Belmont and Swan and the Eastern Metropolitan Regional Council (EMRC), including Ruth Andrew, Dean Cracknell, Sally De La Cruz, Daniel Hanley, Brian Reed and Rachel Thorp; Bassendean, Bayswater, Belmont and Maylands Historical Societies, Ascot Kayak Club, Claughton Reserve Friends Group, Ellis House, Foreshore Environment Action Group, Friends of Ascot Waters and Ascot Island, Friends of Gobba Lake, Maylands Ratepayers and Residents Association, Maylands Yacht Club, Success Hill Action Group, Urban Bushland Council, Viveash Community Group, Swan Chamber of Commerce, Midland Brick and the other community members who participated in the heritage audit community consultation. Special thanks also to Anne Brake, Albert Corunna, Frances Humphries, Leoni Humphries, Oswald Humphries, Christine Lewis, Barry McGuire, May McGuire, Stephen Newby, Fred Pickett, Beverley Rebbeck, Irene Stainton, Luke Toomey, Richard Offen, Tom Perrigo and Shelley Withers for their support in this project. The views expressed in this document are the views of the authors and do not necessarily represent the views of the EMRC. -
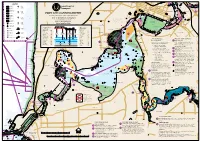
A Boating Guide for the Swan Canning Riverpark
MITCHELL CRESCENT WALCOTT RD 5 Knots WHATLEY Garratt Road Bridge 2.8 Ascot Racecourse STREET Bardon Park STREET GUILDFORD GRANDSTAND VINCENT STREET Maylands Yacht Club City Beach Hospital MAYLANDS ST ANNES ASCOT STREET S.F. ASCOT STREET 8 Knots WATERS 5 Knots BULWER Special Closed Waters Motorised Vessels BEAUFORT SEA SCOUTS FREEWAY STREET Banks Grove Farm Y Y Y Reserve Tranby House Boat Ruins Reserve AERODROME 5 Knots Belmont Park ts BELGRAVIA ST PARADE POWERHOUSE Jetty Ruins o Racecourse Slipway n Overhead Power K MAYLANDS WILLIAM 8 Lines 132kV WEST 11·5 BUNBURY BRIDGE MARKET NEWCASTLE PERTH T STREET S MURRAY ST A E HAY WELLINGTON Windan Bridge Telephone Goongoongup 3.9 STREET Bridge ST BELMONT STREET CAUTION Water STREET Clarkson Reserve STREET Foul Ground Ski Maylands GEORGE 9 Submerged Piles Boat Ramp Area LEGEND 5 knots 270.1° Claisebrook BELMONT LORD 3 5 Knots Cove HIGHWAY Indicates STREET Hardey Park 5 Knot Area safe water MURRAY to the North Bldg (conspic) Belmont Jetty (260) HAY Hospital Boat Shed North STREET Cracknell Park 8 Knot Area N ER Y Closed Waters ST RIVERVALE EA WILLIAM Motorised Vessels 8 Knots for vessels PERTH STREET Gloucester Park over 20m only SHENTON PARK AVE Indicates STREET EAST PERTH BURSWOOD 12 safe water Reservoir BARRACK AVE Barrack St ADELAIDE to the South Jetties WAC Water Ski Area South SWAN AND CANNING RIVERS STREET APBA VICTORIA Speed Foul RIVERSIDE LATHLAIN Non Public Memorial TCE Boat Water Ski Area A boating guide for the Swan Canning Riverpark Kings Park Langley Area Military Exercise Narrows -

Canoeing WA 2014 – 2015
FREE TO MARATHON SERIES Canoeing WA PADDLERS Handbook 2014 – 2015 Thirteenth Edition – September 2014 Canoeing Western Australia (Inc.) PO Box 57, Claremont WA 6910 Phone: (08) 9285 8501 Fax: (08) 9387 8018 Email : [email protected] (Canoeing WA) Email : [email protected] (Marathon Technical Committee) Website: www.wa.canoe.org.au Current Canoeing WA affiliated clubs :-- Ascot Kayak Club, AKC www.ascot.canoe.org.au Phone 0430 561 853 Bayswater PaddleSports Club BPS www.bayswater.canoe.org.au Canning River Canoe Club CRCC www.canningriver.canoe.org.au Champion Lakes Boating Club CLBC www.championlakes.canoe.org.au Phone 0401 311 817 Denmark Riverside Club DRP Look for them on Facebook Phone 9848 2468 Indian Ocean Paddlers IOP www.iop.canoe.org.au Phone Mandurah Paddling Club MPC www.mandurahpaddlingclub.org.au Phone 0400 842 445 Mandurah Ocean Club, MOC www.mandurahoceanclub.com.au Phone 0421 905 132 Perth Canoe Polo Club PCPC www.pcpc.canoe.org.au Phone Sea Kayak WA Club SKWA www.seakayakwa.org.au Phone Swan Canoe Club SCC www.swan.canoe.org.au Phone For the best value in Marathon Racing, Join a Canoeing WA affiliated Club and get Series Registered .. HOW TO ENTER :-- 1. Is your Club membership current? If not please enter as a non-member. 2. Do you have your Australian Canoeing Number? You can search for it via the red box on the Canoeing WA webpage : www.wa canoe.org.au 3. Select Event from Future Events on Canoeing WA webpage. Alternatively link through via the WA State Event Calendar. -

Guppy Book 2017
Who is Paddle WA? Mission Statement Paddle Western Australia represents the whole paddle sports community, comprising of the fol- Paddle WA will maintain the highest lowing core disciplines: sprint racing, slalom, level of expertise and provide oppor- marathon racing, canoe polo, wildwater, sea kay- tunities to all paddlers in competition, aking and ocean racing. recreation, education, training, safety and facilities. Paddle WA gratefully acknowledges the valuable The needs of all paddlers will be rep- help and assistance of its sponsors and support- resented at all levels of government, promoting paddle sports as a positive ers, particularly Healthway, Lotterywest and the life changing physical activity. Department of Local Government, Sport and Cul- tural Industries. Clubs There are 12 Canoeing WA af liated clubs in the Perth Metropolitan area and regional Western Australia. Canoeing clubs can have from 20 to 600 members, with varying skill levels from novice to elite. Clubs regularly run club nights, activities and events for club members to participate in. Details of clubs can be found below with website addresses for further information. ASCOT KAYAK CLUB www.ascotkayakclub.asn.au/ MANDURAH OCEAN CLUB Garvey Park, Ascot www.mandurahoceanclub.com.au BAYSWATER PADDLESPORTS CLUB MANDURAH PADDLING CLUB www.bayswater.canoe.org.au www.mandurahpaddlingclub.org.au AP Hinds Reserve, Bayswater PERTH CANOE POLO CLUB CANNING RIVER CANOE CLUB www.facebook.com/perthcanoepoloclub/ Beatty Park, North Perth www.canningriver.canoe.org.au Fern Road, Riverton PERTH PADDLERS Brown Street Playground, East Perth DENMARK RIVERSIDE CANOE CLUB www.pp.canoe.org.au Fyfe Street, Denmark SEA KAYAK CLUB OF WA CHAMPION LAKES BOATING CLUB www.seakayakwa.asn.au/ www.championlakes.canoe.org.au Champion Lakes Regatta Centre, Armadale SWAN CANOE CLUB www.swan.canoe.org.au INDIAN OCEAN PADDLERS CLUB Johnson Parade, Mosman Park www.iop.asn.au/ WHAT ARE DISCIPLINES ? Disciplines refer to the different “sports” within the greater sport of canoeing, kayaking or, simply, paddling. -

Belmont Foreshore Precinct Plan
BELMONT FORESHORE PRECINCT PLAN May 2018 Above: The foreshore from Cracknell Park’s public jetty, characterised by a linear woodland of tall fringing vegetation (UDLA). Cover: Aerial view of the Swan River’s winding path from Ascot peninsula to Perth City (City of Belmont). CONTENTS Foreword 01 Project Vision Statement and Guiding Principles 02 Introduction 05 Development Control 11 Precinct Description 12 Landscape Character 16 Precinct Strategy 20 Sub-Precinct Plans 24 Further Discussions and Actions 30 Appendix 33 FOREWORD The purpose of the City of Belmont Foreshore Precinct Plan is to provide the City of Belmont, Department of Biodiversity, Conservation and Attractions, Swan River Trust and Western Australian Planning Commission with a detailed planning tool to guide development and uses within the river setting; and ensure that the landscape values of the river system are conserved or enhanced for present and future generations. The plan guides the future use and management of the Belmont foreshore and the development interface with the Parks and Recreation reserve. - 01 - VISION Our vision for the river and its setting is that it displays its true worth as a sustaining resource to Aboriginal society over many millennia and as the foundation of European settlement in Western Australia. We are committed to protecting and enhancing the river by respecting its environmental values, social benefits and cultural significance. We will guide adjacent land use, civic design and development to ensure that the value of the river and its setting to the community is maintained. - Swan and Canning Rivers Precinct Planning Project - Precinct Plan Handbook WHAT DOES THIS ENTAIL? It requires that development respect the benefits and reinforce the setting of the river, its tributaries, floodplains and landscape setting.