Molecular Epidemiology and Risk Factors Assessment of Anaplasma Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Future Strategies for Promoting Tourism and Petroleum Heritage in Khuzestan Province, Iran
Future strategies for promoting tourism and petroleum heritage in Khuzestan Province, Iran Sahar Amirkhani, Neda Torabi Farsani and Homa Moazzen Jamshidi Abstract Sahar Amirkhani and Purpose – Industrial tourism not only strives to preserve industrial heritage, but can also be a strategy for being Neda Torabi Farsani are both familiar with the history of industry and attracting tourists to new destinations. This paper examines the issue of based at the Department of promoting petroleum industrial tourism in the case of Khuzestan, Iran. The research aims at determining Museum and Tourism, Art appropriate strategies for promoting petroleum industrial tourism. University of Isfahan, – Design/methodology/approach The data were analysed through a strengths, weaknesses, opportunities, Isfahan, Iran. and threats (SWOT) model. Homa Moazzen Jamshidi is Findings – The results revealed the competitive strategy as the best. Lastly, strategies such as: concentric based at the Department of diversification, joint venture strategy, conglomerate diversification and horizontal diversification were proposed Economics and Arts as key solutions. The results support the view that establishing an exploratory ecomuseum in the territory of Entrepreneurship, Art Khuzestan Province can be a suitable concentric diversification strategy towards petroleum industrial sustainable tourism in the future. University of Isfahan, Originality/value – The main originality of this paper includes linking tourism with the petroleum (oil and natural Isfahan, Iran. gas) industry -

Analysis of Geographical Accessibility to Rural Health Houses Using the Geospatial Information System, a Case Study: Khuzestan Province, South-West Iran
Acta Medica Mediterranea, 2015, 31: 1447 ANALYSIS OF GEOGRAPHICAL ACCESSIBILITY TO RURAL HEALTH HOUSES USING THE GEOSPATIAL INFORMATION SYSTEM, A CASE STUDY: KHUZESTAN PROVINCE, SOUTH-WEST IRAN FARAHNAZ SADOUGHI1, 2, JAVAD ZAREI1, ALI MOHAMMADI3, HOJAT HATAMINEJAD 4, SARA SAKIPOUR5 1Department of Health Information Management, School of Health Management and Information Science, Iran University of Medical Sciences, Tehran, I.R. Iran - 2Health Management and Economics Research Center, School of Health Management and Information Sciences, Iran University of Medical Sciences, Tehran, I.R. Iran - 3Assistant professor of Health Information Management, Department of Health Information Technology, Paramedical School, Kermanshah University of Medical Sciences, Kermanshah I.R. Iran - 4PhD candidate, Geography and Urban Planning, University of Tehran, Tehran - 5Office of Medical Record and Statistics, Vice-Chancellor for Treatment, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, I.R. Iran ABSTRACT Background: The use of rural health houses is one of the important approaches for delivering health services but, inappro- priate infrastructures and limited resources make it difficult to design and implement plans to enhance and improve health services in rural areas. The aim of this study was to analyze the accessibility to rural health care services in the province of Khuzestan Materials and methods: This applied research was conducted in Khuzestan Province, south-west Iran with a cross-sectional approach in 2014. The population of the study was the villages and rural health houses. All the villages and rural health houses were included in the study without sampling. Descriptive data collected with a checklist from the Statistical Centre of Iran, IT Department of the Management Deputy of the Governor’s Office and Ahvaz Jundishapur and Dezful University of Medical Sciences and spatial data obtained from the national Cartographic Center. -

The Middle Cretaceous – Lower Miocene 3D Petroleum System Modeling of Kupal Oil Field, South West of Iran, Dezful Embayment
Archive of SID Geopersia 10 (1), 2020, PP. 165-194 DOI: 10.22059/GEOPE.2019.281259.648477 The Middle Cretaceous – Lower Miocene 3D petroleum system Modeling of Kupal Oil Field, South West of Iran, Dezful Embayment Mousa Zohrabzadeh1, Hossain Rahimpour–Bonab2*, Mohsen Aleali1 1 Department of Earth Sciences, Science and Research Branch, Islamic Azad University (IAU), Tehran, Iran 2 Department of Geology, Faculty of Sciences, University of Tehran, Tehran, Iran *Corresponding author, e–mail: [email protected] (received: 13/05/2019 ; accepted: 11/12/2019) Abstract The Middle Cretaceous–Lower Miocene petroleum system of the Kupal oil field, located in the Dezful Embayment has been studied. The Kazhdumi and Pabdeh are main source rocks, the Sarvak, Ilam and Asmari formations are reservoir and the Gachsaran formation is the seal rock. According to geochemical analysis, the Kazhdumi and Pabdeh contributed to oil generation and feeding the Asmari and Bangestan reservoirs. Also, the lateral migration from the Kupal to other fields is ruled out but vertical migration led to feeding the Asmari and Bangestan reservoirs. Considering burial history diagrams and modeling of 3D petroleum system in the syncline area between the Marun and Kupal fields, oil generation phases from the Kazhdumi and Pabdeh formations were about 9 and 6 million years ago, respectively. Also in the syncline area between these structures (about 7 and 3 million years ago), the hydrocarbon expulsion started from the Kazhdumi and Pabdeh formations, respectively. Based on 3D hydrocarbon system modeling, in the drainage area of the Kupal oil field, about 51% of the hydrocarbon composition of the Kazhdumi source rock transformed into coke and consumed during the geological periods; but the Pabdeh source rock is remained intact, and only about 18% of the hydrocarbon composition of this formation is transformed into coke. -

Mayors for Peace Member Cities 2021/10/01 平和首長会議 加盟都市リスト
Mayors for Peace Member Cities 2021/10/01 平和首長会議 加盟都市リスト ● Asia 4 Bangladesh 7 China アジア バングラデシュ 中国 1 Afghanistan 9 Khulna 6 Hangzhou アフガニスタン クルナ 杭州(ハンチォウ) 1 Herat 10 Kotwalipara 7 Wuhan ヘラート コタリパラ 武漢(ウハン) 2 Kabul 11 Meherpur 8 Cyprus カブール メヘルプール キプロス 3 Nili 12 Moulvibazar 1 Aglantzia ニリ モウロビバザール アグランツィア 2 Armenia 13 Narayanganj 2 Ammochostos (Famagusta) アルメニア ナラヤンガンジ アモコストス(ファマグスタ) 1 Yerevan 14 Narsingdi 3 Kyrenia エレバン ナールシンジ キレニア 3 Azerbaijan 15 Noapara 4 Kythrea アゼルバイジャン ノアパラ キシレア 1 Agdam 16 Patuakhali 5 Morphou アグダム(県) パトゥアカリ モルフー 2 Fuzuli 17 Rajshahi 9 Georgia フュズリ(県) ラージシャヒ ジョージア 3 Gubadli 18 Rangpur 1 Kutaisi クバドリ(県) ラングプール クタイシ 4 Jabrail Region 19 Swarupkati 2 Tbilisi ジャブライル(県) サルプカティ トビリシ 5 Kalbajar 20 Sylhet 10 India カルバジャル(県) シルヘット インド 6 Khocali 21 Tangail 1 Ahmedabad ホジャリ(県) タンガイル アーメダバード 7 Khojavend 22 Tongi 2 Bhopal ホジャヴェンド(県) トンギ ボパール 8 Lachin 5 Bhutan 3 Chandernagore ラチン(県) ブータン チャンダルナゴール 9 Shusha Region 1 Thimphu 4 Chandigarh シュシャ(県) ティンプー チャンディーガル 10 Zangilan Region 6 Cambodia 5 Chennai ザンギラン(県) カンボジア チェンナイ 4 Bangladesh 1 Ba Phnom 6 Cochin バングラデシュ バプノム コーチ(コーチン) 1 Bera 2 Phnom Penh 7 Delhi ベラ プノンペン デリー 2 Chapai Nawabganj 3 Siem Reap Province 8 Imphal チャパイ・ナワブガンジ シェムリアップ州 インパール 3 Chittagong 7 China 9 Kolkata チッタゴン 中国 コルカタ 4 Comilla 1 Beijing 10 Lucknow コミラ 北京(ペイチン) ラクノウ 5 Cox's Bazar 2 Chengdu 11 Mallappuzhassery コックスバザール 成都(チォントゥ) マラパザーサリー 6 Dhaka 3 Chongqing 12 Meerut ダッカ 重慶(チョンチン) メーラト 7 Gazipur 4 Dalian 13 Mumbai (Bombay) ガジプール 大連(タァリィェン) ムンバイ(旧ボンベイ) 8 Gopalpur 5 Fuzhou 14 Nagpur ゴパルプール 福州(フゥチォウ) ナーグプル 1/108 Pages -

Natural Geographic Features in Dezful and Susa in the Development of Sustainable Tourism
NATURAL GEOGRAPHIC FEATURES IN DEZFUL AND SUSA IN THE DEVELOPMENT OF SUSTAINABLE TOURISM 1HEYDAR LOTFI, 2MARYAM NAHAVANDIAN, 3NEDA GHASEMNIA 1Assistant professor of Garmsar Azad University, Iran 2Master of geography and tourism planning Islamic Azad University of Garmsar ,Iran 3Master of tourism management, Qeshm institute of higher education, Iran E-mail: [email protected], [email protected], [email protected] Abstract-Based on these results, Sasa and Dezful for wheat, barley and rice on irrigated land, respectively, in grades 22, 27 and 21 and on dry land (wheat and barley) has been ranked 28 Which indicates the low efficiency of this activity in the province is compared to other provinces. Some of the causes of this problem, as follows: Salinity and poor soil, more than 60 percent of land in the province (mountainous desert lands) for agricultural activities.Low rate of rainfall. High temperature, resulting in a high rate of evaporation of soil moisture. Poor vegetation cover. Low percentage of literate farmers (based on the results of the General Census of Agriculture., 6/55 percent From illiterate farmers in Khuzestan province and education 9/28 percent of them, the elementary school has been) and as a result of insufficient acquaintance with scientific methods activities of agriculture, horticulture and animal husbandry. Key words- Natural geographic, sustainable tourism,Susa,Dezful I. PROBLEM STATEMENT hand and the loss of productivity at the workplace on the other hand, Weather conditions is one of the main Khuzestan province with an area of 63633 square causes of brain drain (expert and capitalists) from the kilometers between 29 degrees and 57 minutes north province to the provinces that are more favorable latitude and 33 degrees of the equator and 47 degrees weather conditions, respectively. -

Khuzestan Province, Iran
Journal of Hydraulic Structures J. Hydraul. Struct., 2019; 6(1): 90- 104 DOI: 10.22055/jhs.2020.32747.1133 The Impact of outlier detection to estimate groundwater fluctuations using GRACE satellite data; Case Study: Khuzestan Province, Iran Mohammad Mehdi Riyahi1* Mohammad Jafarpour1 Lotfollah Emadali2 Mohammad Ali Sharifi3 Abstract Groundwater aquifers are one of the most significant freshwater resources in the world. Hence, the monitoring of these resources is particularly important for available water resources planning. Piezometric wells have traditionally been used to monitor groundwater. This approach is costly and pointwise, which is not feasible for places with steep topography and mountainous areas. Nowadays, remote sensing techniques are widely used in various fields of engineering as appropriate alternatives to traditional methods. In water resources management, the Gravity Recovery and Climate Experiment (GRACE) satellites can monitor groundwater changes with acceptable accuracies. This paper applied the GRACE satellite data for a 40-month period to assess the variation of the groundwater level in Khuzestan province. The Global Land Data Assimilation System (GLDAS) model was used to counteract the soil moisture effect in final results. The observed data from piezometric wells were pre-processed to detect outliers using the Mahalanobis algorithm in Khuzestan province. At last, the outputs of GRACE were compared with these processed observed data. Despite the relatively small size of the area in question, the results indicated -

Iraq Situation Sources: UNHCR Field Office UNHCR, Global Insight Digital Mapping Elevation © 1998 Europa Technologies Ltd
FF II CC SS SS Capital Armistice Demarcation Line Field Information and Administrative boundary Coordination Support Section UNHCR Representation Main road Division of Operational Services UNHCR Sub office Railway Iraq Situation Sources: UNHCR Field office UNHCR, Global Insight digital mapping Elevation © 1998 Europa Technologies Ltd. UNHCR Presence (Above mean sea level) MoDM, IOM, IDP Working Group C Refugee settlement As of April 2008 3,250 to 4,000 metres Refugee camp 2,500 to 3,250 metres The boundaries and names shown and the designations used on this Town or village of interest 1,750 to 2,500 metres map do not imply official endorsement 1,000 to 1,750 metres Exclusively for internal UNHCR use !! Main town or village or acceptance by the United Nations. 750 to 1,000 metres ((( Secondary town or village Iraq_SituationMapEthnoGroups_A3LC.WOR ((( ((( ((( 500 to 750 metres ((( Andirin !! ((( ((( ((( ((( Hakkâri ((( Yüksekova Kahramanmaras((( ((( ((( Gercus !! ((( ((( !! ((( ((( Kuyulu ((( Savur International boundary ((( Pazarcik((( Golcuk ((( !! 250 to 500 metres ((( !! ((( ((( !! ((( ((( !! ((( ((( !! ((( Bandar-e Anzali !! ((( !! ((( Karakeci OrumiyehOrumiyeh ((( Kozan ((( ((( OrumiyehOrumiyeh ((( Meyaneh ((( ((( ((( ((( !! ((( !! Turkoglu((( Yaylak((( ((( ((( !! Maraghen ((( Boundary of former Kadirli((( !! ((( Akziyaret ((( Derik ((( ((( ((( 0 to 250 metres ((( ((( (((Cizre ((( Bonab !! ((( ((( !! !! ((( ((( ( ((( Mardin Sume`eh Sara !! ((( Kuchesfahan ( ((( ((( ((( ((( SilopiSilopi !! Palestine Mandate Karaisali((( -

Significant Decline of Malaria Incidence in Southwest of Iran (2001–2014)
Hindawi Publishing Corporation Journal of Tropical Medicine Volume 2015, Article ID 523767, 6 pages http://dx.doi.org/10.1155/2015/523767 Research Article Significant Decline of Malaria Incidence in Southwest of Iran (2001–2014) Shokrollah Salmanzadeh,1 Masoud Foroutan-Rad,2 Shahram Khademvatan,3 Sasan Moogahi,1 and Shahla Bigdeli4 1 Health Research Institute, Infectious and Tropical Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran 2Department of Medical Parasitology & Student Research Committee, Faculty of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran 3Cellular and Molecular Research Center and Department of Medical Parasitology and Mycology, Urmia University of Medical Sciences, P.O. Box 571551441, Urmia, Iran 4CDC Department, Deputy of Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran Correspondence should be addressed to Shahram Khademvatan; [email protected] Received 29 June 2015; Revised 28 October 2015; Accepted 28 October 2015 Academic Editor: Carlos E. P. Corbett Copyright © 2015 Shokrollah Salmanzadeh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Iran is considered as one of the malaria endemic countries of the Eastern Mediterranean Region (EMR) and is at risk due to neighboring Afghanistan, Pakistan in the east, and Iraq to the west. Therefore the aim of the present investigation is the evaluation of the trend of malaria distribution during the past decade (2001–2014) in Khuzestan province, southwestern Iran. In this retrospective cross-sectional investigation, blood samples were taken from all malaria suspicious cases who were referred to health centers across Khuzestan province. -
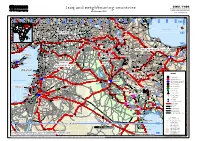
Iraq and Neighbouring Countries Geographic Information and Mapping Unit Population and Geographic Data Section As of November 2003 Email : [email protected]
GIMU / PGDS Iraq and neighbouring countries Geographic Information and Mapping Unit Population and Geographic Data Section As of November 2003 Email : [email protected] Haymana )))) )))) )))) )))) )))) Goradiz ))) )))) Akpinar )))) )))) Tecer )))) Sincan )))) ))) Bank ))) Cheleken )))) )))) )))) ))) ))) )))) )))) )))) Akbenli )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) ))) )))) )))) )))) )))) Sarkisla )))) )))) Hinis ))) Neftechala )))) )))) )))) )))) ))) Karacaoren )))) )))) )))) 33° E 34° E 35° E 36° E )))) 37° E 38° E 39° E 40° E 41° E 42° E 43° E 44° E 45° E 46° E 47° E 48° E 49° E 50° E 51° E 52° E 53° E )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) Uzunlu)))) )))) )))) )))) )))) Caylar )))) )))) )))) )))) Kangal )))) )))) )))) )))) )))) )))) )))) )))) ))) )))) )))) )))) Karaoglan Nakhichevan' ))) ))) ))) )))) )))) )))) )))) ))) ))) Dzhalilabad )))) )))) )))) )))) ))) )))) )))) )))) UU ))) )))) Seker )))) Varto )))) UU ))) ))) )))) )))) UU ))) ))) )))) )))) UU ))) ))) )))) UU ))) ))) )))) UU ))) ))) )))) )))) UU ))) ))) Kirsehir )))) )))) )))) )))) )))) Kadzharan ))) )))) )))) )))) )))) )))) )))) )))) )))) ))) Prishib )))) )))) )))) )))) Hozat)))) Tunceli )))) Sancak )))) ))) )))) )))) )))) )))) )))) )))) )))) )))) ))) )))) )))) )))) )))) )))) Bulanik Paraga ))) )))) )))) )))) Horan )))) )))) )))) )))) )))) Arapkir )))) )))) )))) )))) ))) )))) )))) )))) Ercis )))) )))) ))) Masally )))) )))) )))) )))) 39° N )))) )))) )))) )))) )))) )))) ))) )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) )))) -

Table of Contents · VIEW PER PAGE: · 1 2 SHOWING 1-50 of 52 CROSS
O x 2 Table of Contents · VIEW PER PAGE: · 1 2 SHOWING 1-50 of 52 · OAK CROSS-REFERENCE See BALŪṬ. · ʿ OBAYD ZĀKĀNI DANIELA MENEGHINI a Persian poet from the Mongol period (d. ca. 770/1370), renowned above all for his satirical poems. · OBOLLA C. EDMUND BOSWORTH a port of Lower Iraq during the classical and medieval Islamic periods. · OḠUZ KHAN NARRATIVES İLKER EVRIM BINBAŞ The Tāriḵ-e Oḡuz begins with a short genealogical and topographical introduction connecting the family of Oḡuz to that of Japheth, or Öljey/Oljāy Khan, as he is called in the text, and his son Dib Yāwqu Khan, who lived nomadic life around the lakes of Issyk-Kul and Balkhash. This Article Has Images/Tables. · OHRMAZD CROSS-REFERENCE Middle Persian name of the supreme deity in Zoroastrianism. See AHURA MAZDĀ. · OIL AGREEMENTS IN IRAN PARVIZ MINA (1901-1978): their history and evolution. The history of Iranian oil agreements began with an unprecedented concession granted by Nāṣer-al- Din Shah in 1872 to Baron Julius de Reuter. · OIL INDUSTRY MULTIPLE AUTHORS i. Petroleum and its Products. ii. Iran's Oil and Gas Resources · OIL INDUSTRY I. PETROLEUM AND ITS PRODUCTS A. BADAKHSHAN AND F. NAJMABADI The first requisite for an oil or a gas field is a reservoir: a rock formation porous enough to contain oil or gas and permeable enough to allow their movement through it. This Article Has Images/Tables. · OIL INDUSTRY II. IRAN’S OIL AND GAS RESOURCES A. BADAKHSHAN AND F. NAJMABADI The Iranian oil industry is the oldest in the Middle East. -

Iraq SB As of 11Jan08.Pub
UNHCR Iraq Situation Supplementary Appeal P.O. Box 2500 1211 Geneva 2 Switzerland : +41 22 739 79 56 : +41 22 739 73 58 : [email protected] You, too, can help refugees. Visit our website at 2008 Iraq Situation Supplementary Appeal FICSS in DOS Iraq Atlas Map Field Information and Coordination Support Section As of December 2007 Division of Operational Services Email : [email protected] Pazarcik Golcuk Karakeci Senkoy Meyaneh Turkoglu Yaylak Maraghen Derik Heshajeyn Viransehir Idil Cizre Bonab Mardin Sanliurfa Kuchesfahan Kapakli SilopiSilopi SilopiSilopi Gaziantep Kiziltepe Zakho Malek Kandi Rud Sar Al 'Amadiyah Al Qamesheli Nizip Suruc TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY TURKEY Sazgin Naqadeh Miandoab Ceylanpinar DohukDohuk DohukDohuk Mahabad Kilis Akcakale ZivehZiveh ZivehZiveh Sa'in Dezh Zanjan Tall Tamir Saluq Manbij Bukan ElEl HassakeHassake Tall 'Afar DilzehDilzeh ISLAMIC REPUBLIC ISLAMIC REPUBLIC ISLAMICISLAMIC REPUBLICREPUBLIC ISLAMICISLAMIC REPUBLICREPUBLIC ElEl HolHol ISLAMICISLAMIC REPUBLICREPUBLIC ISLAMICISLAMIC REPUBLICREPUBLIC ISLAMIC REPUBLIC ElEl HolHol ISLAMICISLAMIC REPUBLICREPUBLIC Aleppo Qazvin Saqqez yhanli Dayr Hafir ErbilErbil BazilehBazileh BazilehBazileh OF IRAN As Safirah Al Quwayr Takestan SoltaniehSoltanieh Iraq_Atlas_A3LC.WOR KawaKawa Baneh SoltaniehSoltanieh KawaKawa Bijar Idlib Ar Raqqah Shal Garm Ab Najmabad Ariha MakhmourMakhmour Alla Kabud Abu ad Duhur Estehard -

Effects of Climate Variables on the Incidence of Scorpion Stings in Iran for Five Years
RESEARCH OPEN ACCESS ISSN 1678-9199 www.jvat.org Effects of climate variables on the incidence of scorpion stings in Iran for five years Ahmad Ghorbani1, Behzad Mansouri2, Masoumeh Baradaran1* 1Toxicology Research Center, Medical Basic Sciences Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. 2Department of Statistics, Shahid Chamran University of Ahvaz, Ahvaz, Iran. Abstract Background: Although scorpionism is recorded worldwide, some regions such as Iran present a higher incidence. Due to the great prevalence of scorpion stings in Khuzestan province, southwestern Iran, the present study examined the relationship between different climate parameters and the scorpion sting rate in this area from April 2010 to March 2015. Methods: In this cross-sectional descriptive-analytical study, we considered all scorpion sting cases recorded in the Department of Infectious Diseases, Ahvaz Jundishapur University of Medical Sciences. Data were analyzed using statistics, frequency distribution Keywords: and Pearson’s correlation coefficient. Scorpion stings Results: A total of 104,197 cases of scorpion stings was recorded from 2010 to 2015. Climate factors The cumulative incidence of scorpion sting was 2.23%. The spatial distribution Scorpionism of scorpion stings showed that most cases occurred in the Dehdez district (4,504 Khuzestan province scorpion stings/100,000 inhabitants) and the Masjed Soleyman county (4,069 scorpion stings/100,000 inhabitants). A significant association was found between climate factors Iran (temperature, evaporation rate, sunshine duration, humidity, and precipitation) and the scorpion sting rate. An increase in rainfall and humidity coincided with a reduction in scorpion stings whereas an increase in temperature, evaporation, and sunshine duration was accompanied by a growth of scorpion stings.