Fy /S Affortney Égy E.ARRY D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of Aluminium As Oxide
DETERMINATION OF ALUMINIUM AS OXIDE By William Blum CONTENTS Page I. Introduction 515 II. General principles 516 III. Historical 516 IV. Precipitation of aluminium hydroxide. 518 1. Hydrogen electrode studies 518 (a) The method 518 (b) Apparatus and solutions employed 518 (c) Results of hydrogen electrode experiments 519 (d) Conclusions from hydrogen electrode experiments 520 2. Selection of an indicator for denning the conditions of precipita- '. tion . 522 3. Factors affecting the form of the precipitate 524 4. Precipitation in the presence of iron 525 V. Washing the precipitate . 525 VI. Separation from other elements 526 VII. Ignition and weighing of the precipitate 528 1. Hygroscopicity of aluminium oxide 529 2. Temperature and time of ignition 529 3. Effect of ammonium chloride upon the ignition 531 VIII. Procedure recommended 532 IX. Confirmatory experiments 532 X. Conclusions '534 I. INTRODUCTION Although a considerable number of precipitants have been pro- posed for the determination of aluminium, direct precipitation of aluminium hydroxide by means of ammonium hydroxide, fol- lowed by ignition to oxide, is most commonly used, especially if no separation from iron is desired, in which latter case special methods must be employed. While the general principles involved in this determination are extremely simple, it has long been recog- nized that certain precautions in the precipitation, washing, and ignition are necessary if accurate results are to be obtained. While, however, most of these details have been studied and dis- cussed by numerous authors, it is noteworthy that few publica- tions or textbooks have taken account of all the factors. In the 515 ; 516 Bulletin of the Bureau of Standards [Voi.i3 present paper it seems desirable, therefore, to assemble the various recommendations and to consider their basis and their accuracy. -

Safety Data Sheet
SAFETY DATA SHEET FennoFloc A 19 Ref. /US/EN Revision Date: 02/09/2017 Previous date: 02/09/2017 Print Date:04/20/2017 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING Product information Product name FennoFloc A 19 Recommended use of the chemical and restrictions on use Use of the Substance/Mixture Recommended restrictions on use There are no uses advised against. Supplier's details Kemira Chemicals, Inc. 1000 Parkwood Circle, Suite 500 30339 Atlanta USA Telephone+17704361542, Telefax. +17704363432 HEAD OFFICE Kemira Oyj P.O. Box 330 00101 HELSINKI FINLAND Telephone +358108611 Telefax +358108621124 Emergency telephone number CHEMTREC: 1-800-424-9300 CANUTEC: 1-613-996-6666 2. HAZARDS IDENTIFICATION Classification of the substance or mixture Corrosive to metals; Category 1; May be corrosive to metals.; Serious eye damage; Category 1; Causes serious eye damage.; GHS-Labelling 1/14 SAFETY DATA SHEET FennoFloc A 19 Ref. /US/EN Revision Date: 02/09/2017 Previous date: 02/09/2017 Print Date:04/20/2017 Hazard pictograms : Signal word : Danger Hazard statements : Hazard statements: H290 May be corrosive to metals. H318 Causes serious eye damage. Precautionary statements : Prevention: P234 Keep only in original container. P264 Wash face, hands and any exposed skin thoroughly after handling. P280 Wear protective gloves/ eye protection/ face protection. Response: P390 Absorb spillage to prevent material damage. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P310 Immediately call a POISON CENTER or doctor/ physician. Storage: P406 Store in corrosive resistant container with a resistant inner liner. -

A Critical Appraisal and Review of Aluminium Chloride Electrolysis for the Production of Aluminium
Bulletin of Eledrochemistry 1 (5) Sep.-Od. 1985, pp. 483488 A CRITICAL APPRAISAL AND REVIEW OF ALUMINIUM CHLORIDE ELECTROLYSIS FOR THE PRODUCTION OF ALUMINIUM C N KANNAN and PS DESIKAN Central Electrochemical Research Institute, Karaikudi - 623 006, ABSTRACT This paper is an attempt to examine the current state of art of aluminium chloride electrolysis through a review of all the available published literature and patents so that this could help the formulation of the plans of work for any serious R&D effort to develop the chloride te&nolcgy in this country. Even though the development of technology for aluminium chloride electrolysis is being carried out in a big way by ALCOA and a few other multinational companies for the past several years, many of the data and information are lacking in published literature and the answers to various critical questions have to be found only through inferences from the meagre information available in patents. It was therefore thought fit to undertake a thorough review of all the basic applied and R&Dwork that are reported in this field and critically assess the various problems to be tackled to evolve a viable technology. This review confines itself to the electrolytic aspect and those relating to the material preparation will be taken up separately later. Key words : Production of aluminium. Molten salt electrolysis, chlorination of alumina INTRODUCTION The most obvious advantages of the aluminium chloride smelting are 131 : he need to develop a new technology for aluminium metal production 1. Substantially lower working temperature (700°C) compared to Hall- T has been felt very much in the major aluminium producing countries Heroult cell (980°C). -

Aluminum Chloride 7446-70-0 >95
SAFETY DATA SHEET Creation Date 10-Sep-2010 Revision Date 27-May-2019 Revision Number 6 1. Identification Product Name Aluminium chloride Cat No. : AC217460000; AC217460025; AC217460050; AC217461000; AC217465000 CAS-No 7446-70-0 Synonyms Aluminium trichloride Recommended Use Laboratory chemicals. Uses advised against Food, drug, pesticide or biocidal product use. Details of the supplier of the safety data sheet Company Fisher Scientific Acros Organics One Reagent Lane One Reagent Lane Fair Lawn, NJ 07410 Fair Lawn, NJ 07410 Tel: (201) 796-7100 Emergency Telephone Number For information US call: 001-800-ACROS-01 / Europe call: +32 14 57 52 11 Emergency Number US:001-201-796-7100 / Europe: +32 14 57 52 99 CHEMTREC Tel. No.US:001-800-424-9300 / Europe:001-703-527-3887 2. Hazard(s) identification Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Skin Corrosion/Irritation Category 1 B Serious Eye Damage/Eye Irritation Category 1 Specific target organ toxicity (single exposure) Category 3 Target Organs - Respiratory system. Label Elements Signal Word Danger Hazard Statements Causes severe skin burns and eye damage May cause respiratory irritation ______________________________________________________________________________________________ Page 1 / 7 Aluminium chloride Revision Date 27-May-2019 ______________________________________________________________________________________________ Precautionary Statements Prevention Do not breathe dust/fume/gas/mist/vapors/spray Wash face, hands and any exposed skin thoroughly after handling Wear protective gloves/protective clothing/eye protection/face protection Use only outdoors or in a well-ventilated area Response Immediately call a POISON CENTER or doctor/physician Inhalation IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing Skin IF ON SKIN (or hair): Take off immediately all contaminated clothing. -

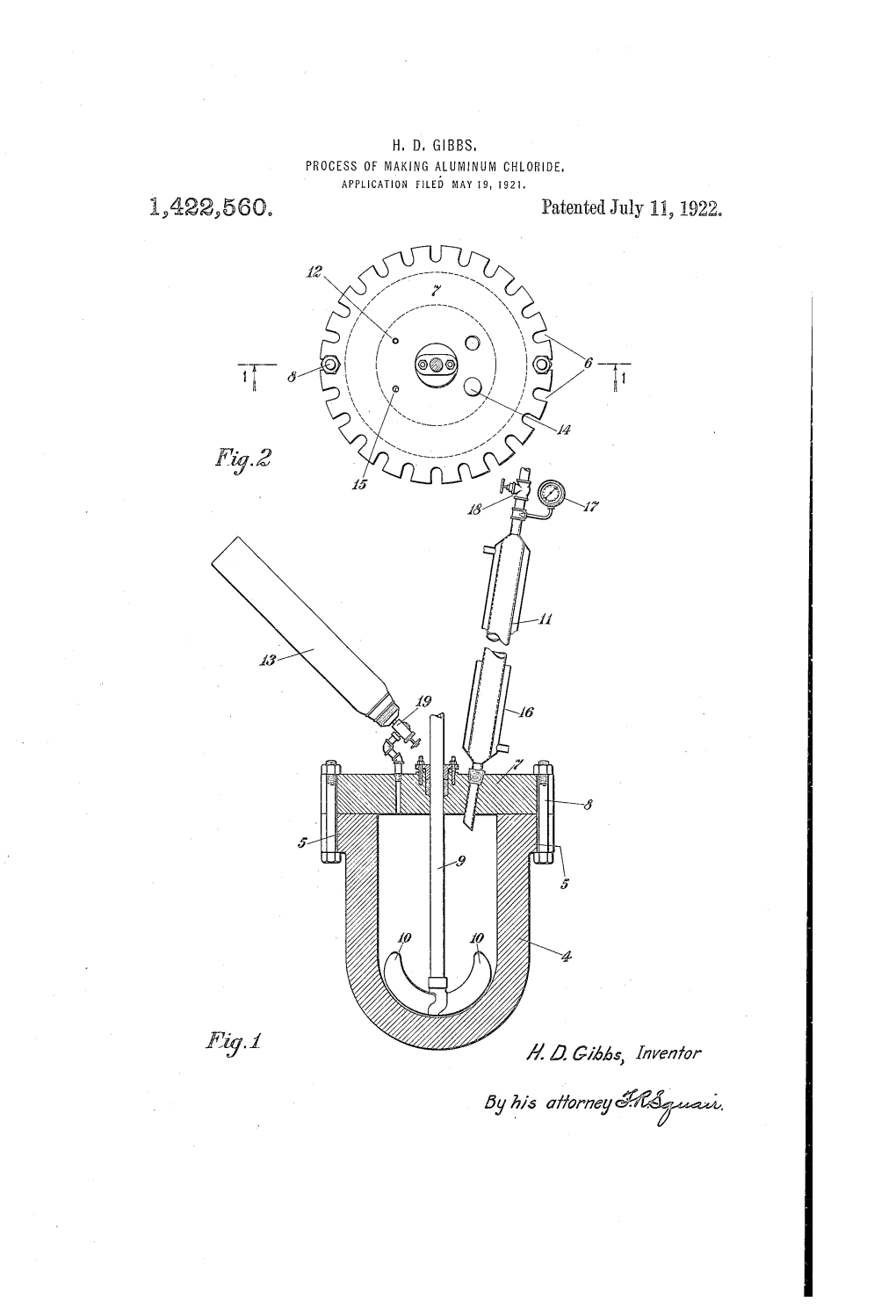
United States Patent Office 2 1
2,934,550 Patented Apr. 26, 1960 United States Patent Office 2 1. phenyl or vinyl groups. By “high molecular weight" I mean of molecular weight not less than about 1000. 2,934,550 The material reacted may be in the form of a poly PREPARATION OF CYCLIC METHYLPOLYSILox. siloxane elastomer composition which will, of course, nor ANES BY REACTING ORGANOPOLYSILOXANES mally contain one or more fillers, pigments, etc, or may PHORUSWITH HALDES OF ALUMINUM oR PHOS be in the form of a polysiloxane gum such as is used in the preparation of polysiloxane elastomers. Partially James Jack, Troon, Scotland, assignor to Imperial Chemi cured polysiloxane compositions may also be used and in tionAff." of Great Britain 9 EEE, England, a corpora addition, other high molecular weight linear polysiloxanes 0 may be treated by this process. The advantages of the No Drawing. Application December 8, 1958 process are, however, of most value in the recovery of Serial No. 778,609 elastomersHalides ofand aluminium gums. and phosphorus which may be Claims priority, application Great Britain used in this process include aluminium chloride, alumin January 17, 1958 ium fluoride, aluminium bromide, phosphorus trichlo 5 ride, phosphorus pentachloride and phosphorus oxychlo 8 Claims. (CI. 260-448.2) ride. Aluminium chloride is, however, preferred, since This invention relates to the treatment of high molecu it is relatively inexpensive and does not tend to contami lar weight Substantially linear polysiloxanes and to the nate the products. preparation of low molecular weight cyclic polysiloxanes 20 The process of my invention is normally carried out therefrom. by heating the reactants together in a vessel adapted for High molecular weight linear organopolysiloxanes, for distilation and the low molecular weight cyclic poly example, such as polysiloxane elastomers and gums used siloxanes are distilled off as the reaction proceeds, the for the production thereof, are widely known and used. -

Ep 1831144 B1
(19) & (11) EP 1 831 144 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07C 37/50 (2006.01) C07C 39/17 (2006.01) 17.08.2011 Bulletin 2011/33 C07C 39/26 (2006.01) (21) Application number: 05816593.7 (86) International application number: PCT/HU2005/000128 (22) Date of filing: 07.12.2005 (87) International publication number: WO 2006/061666 (15.06.2006 Gazette 2006/24) (54) A NEW PROCESS FOR THE PREPARATION OF PHENOLIC HYDROXY-SUBSTITUTED COMPOUNDS NEUES VERFAHREN ZUR HERSTELLUNG PHENOLISCHER HYDROXY SUBSTITUIERTER VERBINDUNGEN PROCEDE D’ELABORATION DE COMPOSES PHENOLIQUES A SUBSTITUTION HYDROXY (84) Designated Contracting States: • BORBÉLY, László AT BE BG CH CY CZ DE DK EE ES FI FR GB GR H-2510 Dorog (HU) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • HAÁSZ, Ferenc SK TR H-2500 Esztergom (HU) Designated Extension States: • JEKKEL, Péter AL BA HR MK YU H-2509 Esztergom-Kertváros (HU) (30) Priority: 08.12.2004 HU 0402530 (74) Representative: HOFFMANN EITLE 11.11.2005 HU 0501044 Patent- und Rechtsanwälte Arabellastraße 4 (43) Date of publication of application: 81925 München (DE) 12.09.2007 Bulletin 2007/37 (56) References cited: (73) Proprietor: Richter Gedeon Nyrt. US-A- 4 339 614 1103 Budapest (HU) • MANABU NODE ET.AL.: "Hard acid and soft (72) Inventors: nucleophile system. 2. Demethylation of methyl • VASS, András ethers of alcohol and phenol with an aluminium H-8200 Veszprém (HU) halide-thiolsystem" J. ORG. CHEM., vol. 45, 1980, • DUDÁS, József pages 4275-4277, XP002379759 cited in the H-8200 Veszprém (HU) application Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Title Reaction of 1,2-Dichloroethane with Toluene Author(S) Kunichika, Sango; Oka, Shinzaburo; Sugiyama, Takashi Citation Bullet
Title Reaction of 1,2-Dichloroethane with Toluene Author(s) Kunichika, Sango; Oka, Shinzaburo; Sugiyama, Takashi Bulletin of the Institute for Chemical Research, Kyoto Citation University (1971), 48(6): 276-282 Issue Date 1971-03-24 URL http://hdl.handle.net/2433/76346 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Bull. Inst. Chem. Res., Kyoto Univ., Vol. 48, No. 6, 1970 Reaction of 1,2-Dichloroethane with Toluene Sango KUNICHIKA, Shinzaburo OKA and Takashi SUGIYAMA* ReceivedDecember 15, 1970 Friedel-Crafts reaction of toluene with 1,2-dichloroethane was studied. The effective catalysts were aluminium bromide, aluminium chloride, gallium chloride and zirconium chloride. Other Lewis acids examined were inactive. The conversion of 1,2-dichloroethane was completed above 50°C and 40% at 25°C with aluminium chloride. Polycondensationproducts were mainly formed via disproportionation of ditolylethanes. Relationship between the isomer distribution and the conver- sion or the reaction time was clarified. INTRODUCTION The reaction of 1,2-dichloroethane with toluene in the presence of Friedel-Crafts type catalyst gives a mixture of six isomers of 1,2-ditolylethane as follows'" : ~~CHCHz OOCHzCHZYJCH3 CH3CH3 CH,J~(%)CH3 CH3 ~~()J) CH3 l) + C1CH,CH,C1+CH,CH,CHzCHz0 CH3SII7 CrCH,CH,0CH3.©CH,CH,CH, -CH,CHt It was described in the previous paper" that these six pure isomers were synthe- sized and an analytical method of these isomers by gaschromatography was established. In the present paper the effective catalysts for this reaction were searched, and the influence of the reaction conditions on the conversion of 1,2-dichloroethane and on the isomer distribution were also examined. -

A Regioselective One Pot Synthesis and Synthetic
A Regioselective One Pot Synthesis and Synthetic Applications of Cyanodeoxy Sugars by Cyanotrimethylsilane [1] Syed Najam-ul-Hussain Kazmia, Zaheer Ahmed3, Abdul Malik3 *, Nighat Afzab, Wolfgang Voelterc a H. E. J. Research Institute of Chemistry, University of Karachi, Karachi 75270, Pakistan b Pharmaceutical and Fine Chemical Division, P.C.S.I.R. Laboratories, Karachi 75280, Pakistan c Abteilung für Physikalische Biochemie, Physiologisch-chemisches Institut der Universität Tübingen. Hoppe-Seyler-Straße 4, 72076 Tübingen. Germany Z. Naturforsch. 50b, 294-302 (1994); received June 28, 1994 Anhydro Sugars, Cyanodeoxy Sugars, Epoxy Ring Opening, Branched Chain Pyranoses, Cyanotrimethylsilane The regioselective epoxide opening of different 2,3-anhydropyranoses by cyanotrimethyl silane is investigated. The isolated cyanodeoxy pyranoses allow easy access to the correspond ing branched-chains aminomethyl sugars by lithium aluminium hydride reduction or sugar amides by controlled acid hydrolysis. Introduction described by them and involves initial formation Cyanodeoxy sugars assume a key role in the of C12A1CN species by the reaction of cyanotri syntheses of C-glycosyl compounds and branched- methylsilane and aluminium chloride. The reac chain sugars [2-5] besides serving as precursors tion of the C12A1CN species with oxiranes gives for most of the naturally occurring C-nucleoside a cyano-aluminated product [12] that undergoes antibiotics and their analogs [2-6], One method fra«s-metallation with cyanotrimethylsilane to give employed, amongst others, for the synthesis of trimethylsilyloxy nitrile with the regeneration of cyanodeoxy sugars involves cleavage of the oxir- the A1C13 species. An alternate mechanism is also ane ring in 2,3-anhydropyranosides by a variety of proposed [13] in which a complex is formed be nitrile containing reagents [7], The epoxides of tween the Lewis acid and the oxygen of the monocyclic sugars have a flexible half-chair con strained ether. -

Opinion of the Scientific Committee on Consumer Safety on O
SCCS/1613/19 Final Opinion Version S Scientific Committee on Consumer Safety SCCS OPINION ON the safety of aluminium in cosmetic products Submission II The SCCS adopted this document at its plenary meeting on 03-04 March 2020 SCCS/1613/19 Final Opinion Opinion on the safety of aluminium in cosmetic products – submission II ___________________________________________________________________________________________ ACKNOWLEDGMENTS Members of the Working Group are acknowledged for their valuable contribution to this Opinion. The members of the Working Group are: For the preliminary and the final versions SCCS members Dr U. Bernauer Dr L. Bodin (Rapporteur) Prof. Q. Chaudhry (SCCS Chair) Prof. P.J. Coenraads (SCCS Vice-Chair and Chairperson of the WG) Prof. M. Dusinska Dr J. Ezendam Dr E. Gaffet Prof. C. L. Galli Dr B. Granum Prof. E. Panteri Prof. V. Rogiers (SCCS Vice-Chair) Dr Ch. Rousselle Dr M. Stepnik Prof. T. Vanhaecke Dr S. Wijnhoven SCCS external experts Dr A. Koutsodimou Dr A. Simonnard Prof. W. Uter All Declarations of Working Group members are available on the following webpage: http://ec.europa.eu/health/scientific_committees/experts/declarations/sccs_en.htm This Opinion has been subject to a commenting period of a minimum eight weeks after its initial publication (from 16 December 2019 until 17 February 2020). Comments received during this time period are considered by the SCCS. For this Opinion, some changes occurred, in particular in sections 1, 3.2, 3.3.4.5, 3.3.8.1, 3.5, as well as in related discussion parts and conclusion (question 1). The list of references has also been updated. -

M/S. KRIMASIL PVT. LTD, GIDC, ANKLESHWAR
M/s. KRIMASIL PVT. LTD, GIDC, ANKLESHWAR ANNEXURE-1 TOR • Standard TOR of sector specific organic synthetic chemicals prescribed by MoEFand CCE will be followed. • To use Baseline monitoring details of A-One Chemicals for the month Dec-2016 to Feb-2017. M/s. Jyoti Om Chemical Research Centre Pvt. Ltd. 1 M/s. KRIMASIL PVT. LTD, GIDC, ANKLESHWAR ANNEXURE-2 LIST OF DIRECTORS Sr. Name Present Residential Designation Contact No. No Address 1. Mr. Nagjibhai Madhabhai Sanskruti Cottage, Director 98241 21717 Ladumor 10, Advance Co- op.Hsg. Society, GIDC Ankleshwar- 393 002. 2. Mrs. Godavari Nagjibhai Sanskruti Cottage, Director 99244 75999 Ladumor 10, Advance Co- op.Hsg. Society, GIDC Ankleshwar- 393 002. 3. Mr. Dhruvesh Nagjibhai Sanskruti Cottage, Director 78744 23122 Ladumor 10, Advance Co- op.Hsg. Society, GIDC Ankleshwar- 393 002. M/s. Jyoti Om Chemical Research Centre Pvt. Ltd. 2 M/s. KRIMASIL PVT. LTD, GIDC, ANKLESHWAR ANNEXURE-3 (A) SITE LAYOUT N . RESIN & PIGMENTS BLANK PLOT LTD PVT. KRIMASIL SPC LIFE SCIENCE GIDC ROAD . VIHITA CHEM PVT. LTD M/s. Jyoti Om Chemical Research Centre Pvt. Ltd. 3 M/s. KRIMASIL PVT. LTD, GIDC, ANKLESHWAR ANNEXURE-3 (B) PLANT LAYOUT M/s. Jyoti Om Chemical Research Centre Pvt. Ltd. 4 M/s. KRIMASIL PVT. LTD, GIDC, ANKLESHWAR NNEXURE-4 LIST OF PRODUCT Sr. Name of product Existing capacity in Proposed Total capacity in MT/Month as per capacity in MT/Month No AWH-84549 MT/Month 1. PIGMENT GREE-7 3 MT/Month 147 MT/Month 150 MT/Month (Copper Phthalocyanine Green) Poly Aluminium Chloride --- 750 MT/Month 750 MT/Month AND /OR 2. -

Facile Iron(III) Chloride Hexahydrate Catalyzed Synthesis of Coumarins
General Papers ARKIVOC 2015 (v) 248-258 Facile iron(III) chloride hexahydrate catalyzed synthesis of coumarins Saisuree Prateeptongkum,*a Nongnaphat Duangdee,b and Panumart Thongyooa aDepartment of Chemistry, Faculty of Science & Technology, Thammasat University, 99 Moo 18 Paholyothin Road, Klong Luang, Rangsit, Prathumthani 12121, Thailand bDrug Discovery and Development Center, Thammasat University, 99 Moo 18 Paholyothin Road, Klong Luang, Rangsit, Prathumthani 12121, Thailand E-mail: [email protected] DOI: http://dx.doi.org/10.3998/ark.5550190.p008.947 Abstract A practical and inexpensive synthesis of coumarins, from phenols and -keto esters, via the Pechmann reaction catalyzed by 10 mol% of FeCl3·6H2O is described. The reaction was applied to transform phenols and -keto esters into the corresponding coumarins in moderate to excellent yields. Keywords: Pechmann reaction, coumarin, hydrated Fe(III) chloride, phenols-keto esters Introduction Coumarins are one of the important structural units and widely found in nature.1-4 They show diverse biological and pharmacological activities ranging from antimicrobial, anti-arrhythmic, antitumor, antifungal, anti-HIV, anti-osteoporosis to anti-inflammatory.5-12 Apart from their pharmaceutical applications,13-14 coumarins have been used as additives in foods, perfumes and cosmetics as well as in the preparation of optical brighteners, laser dyes, fluorescent labels and nonlinear optical chromophores.15-19 The Pechmann reaction20 is known as one of the most valuable methods for the synthesis of -

Secondary Aluminium-Iron (III) Chloride Batteries with a Low Temperature Molten Salt Electrolyte
JOURNAL OF APPLIED ELECTROCHEMISTRY 22 (1992) 230-234 Secondary aluminium-iron (III) chloride batteries with a low temperature molten salt electrolyte F. M. DONAHUE*, S. E. MANCINI, L. SIMONSEN Minotaur Technologies, Ann Arbor, MI 48103-4115, USA Received 11 January 1991; revised 7 May 1991 Secondary aluminium-iron (III) chloride batteries using a low temperature molten salt electrolyte were constructed and tested. Discharge current densities were in the range 5 to 100mA (~ 1 to 20mAcm-2; ~ C/4 to 5C); charging currents were 5mA (C/4 toC/2). Utilization of the positive electrode reactant was low due to the discharge rates and loading procedure. The mode for self discharge was dissolution of the positive electrode reactant and transport to the aluminium negative electrode where it reacted. 1. Introduction Primary A1/WC16 and A1/Br2 cells were studied in basic MEIC melts [2]. Secondary batteries with Low temperature molten salts based on mixtures MEIC-containing electrolytes were studied which also of organic salts (e.g. butylpyridinium chloride, incorporated the characteristics of concentration cells 1-methyl-3-ethylimidazolium chloride (MEIC), and [2]. A1/C12 primary and secondary configurations were 1,2-dimethyl-3-propylimidazolium chloride (DMPIC)) tested in basic and acidic melts, respectively; solubility and aluminium chloride have been proposed as bat- of chlorine in the electrolyte caused significant self- tery electrolytes [1-6]. Binary solutions of these types discharge at the negative electrode in both electrolytes are normally called basic (organic salt-rich), neutral and, in the case of the acidic melts, resulted in chlori- (equimolar), and acidic (AIC13-rich). nation of the organic cation [4, 11].