Renovascular Disease in Children
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 20 *Lecture Powerpoint the Circulatory System: Blood Vessels and Circulation
Chapter 20 *Lecture PowerPoint The Circulatory System: Blood Vessels and Circulation *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The route taken by the blood after it leaves the heart was a point of much confusion for many centuries – Chinese emperor Huang Ti (2697–2597 BC) believed that blood flowed in a complete circuit around the body and back to the heart – Roman physician Galen (129–c. 199) thought blood flowed back and forth like air; the liver created blood out of nutrients and organs consumed it – English physician William Harvey (1578–1657) did experimentation on circulation in snakes; birth of experimental physiology – After microscope was invented, blood and capillaries were discovered by van Leeuwenhoek and Malpighi 20-2 General Anatomy of the Blood Vessels • Expected Learning Outcomes – Describe the structure of a blood vessel. – Describe the different types of arteries, capillaries, and veins. – Trace the general route usually taken by the blood from the heart and back again. – Describe some variations on this route. 20-3 General Anatomy of the Blood Vessels Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Capillaries Artery: Tunica interna Tunica media Tunica externa Nerve Vein Figure 20.1a (a) 1 mm © The McGraw-Hill Companies, Inc./Dennis Strete, photographer • Arteries carry blood away from heart • Veins -

The Circulatory System
TheThe CirculatoryCirculatory SystemSystem Xue Hui DepartmentDepartment ofof HistologyHistology && EmbryologyEmbryology TheThe CirculatoryCirculatory SystemSystem Cardiovascular system (blood vascular system) Heart Artery Capillary Vein Lymphatic vascular system Lymphatic capillary Lymphatic vessel Lymphatic duct II GeneralGeneral structurestructure ofof thethe bloodblood vesselsvessels Tunica intima Tunica media Tunica adventitia Drawing of a medium-sized muscular artery, showing its layers. II GeneralGeneral structurestructure ofof thethe bloodblood vesselsvessels IIII ArteryArtery Large artery Medium-sized artery Small artery Arteriole II Artery LargeLarge arteryartery Structure Tunica intima Tunica media 40-70 layers of elastic lamina Smooth muscle cells, collagenous fibers Tunica adventitia Function Carry the blood from the heart to the middle arteries Tunica Tunica intima intima Tunica Tunica media media Tunica Tunica adventitia adventitia Transverse sections showing part of a large elastic artery showing a well developed tunica media containing several elastic laminas. II Artery MediumMedium--sizedsized arteryartery Structure Tunica intima: clear internal elastic membrane Tunica media: 10-40 layers of smooth muscle cells Tunica adventitia: external elastic membrane Function Regulate the distribution of the blood to various parts of the body Tunica Internal intima elastic membrane Tunica media External elastic membrane Tunica adventitia II Artery SmallSmall arteryartery Structure characteristic Diameter:0.3-1mm Tunica intima: clear -

Infliximab, a TNF-Α Inhibitor, Reduces 24-H Ambulatory
Journal of Human Hypertension (2014) 28, 165–169 & 2014 Macmillan Publishers Limited All rights reserved 0950-9240/14 www.nature.com/jhh ORIGINAL ARTICLE Infliximab, a TNF-a inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients S Yoshida1, T Takeuchi1, T Kotani1, N Yamamoto1, K Hata1, K Nagai1, T Shoda1,STakai2, S Makino1 and T Hanafusa1 Tumour necrosis factor-alpha (TNF-a) is an important mediator in the pathogenesis of rheumatoid arthritis (RA) and hypertension. TNF-a inhibitors improve clinical symptoms and inhibit joint destruction in RA, but their effect on blood pressure (BP) has not been fully investigated. We measured 24-h BP using an ambulatory BP monitor in 16 RA patients treated with a TNF-a inhibitor, infliximab, to investigate its influence on BP and its association with the regulatory factors of BP and renin-angiotensin-aldosterone and sympathetic nervous systems. Infliximab significantly reduced the 24-h systolic BP (SBP) from 127.4±21.8 to 120.1±23.4 mm Hg (Po0.0001). Particularly, morning BP (0600–0800 h) decreased from 129.7±19.7 to 116.9±13.4 mm Hg (Po0.0001), and daytime BP decreased from 131.8±15.1 to 122.5±13.7 mm Hg (Po0.0001). Infliximab significantly reduced the plasma level of norepinephrine and plasma renin activity (PRA) (from 347.5±180.7 to 283.0±181.8 pg ml À 1 and 2.6±2.7 to 2.1±2.9 ng ml À 1 h À 1, respectively) but did not significantly reduce the plasma levels of dopamine and epinephrine. -

Anatomy Review: Blood Vessel Structure & Function
Anatomy Review: Blood Vessel Structure & Function Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction • The blood vessels of the body form a closed delivery system that begins and ends at the heart. Page 2. Goals • To describe the general structure of blood vessel walls. • To compare and contrast the types of blood vessels. • To relate the blood pressure in the various parts of the vascular system to differences in blood vessel structure. Page 3. General Structure of Blood Vessel Walls • All blood vessels, except the very smallest, have three distinct layers or tunics. The tunics surround the central blood-containing space - the lumen. 1. Tunica Intima (Tunica Interna) - The innermost tunic. It is in intimate contact with the blood in the lumen. It includes the endothelium that lines the lumen of all vessels, forming a smooth, friction reducing lining. 2. Tunica Media - The middle layer. Consists mostly of circularly-arranged smooth muscle cells and sheets of elastin. The muscle cells contract and relax, whereas the elastin allows vessels to stretch and recoil. 3. Tunica Adventitia (Tunica Externa) - The outermost layer. Composed of loosely woven collagen fibers that protect the blood vessel and anchor it to surrounding structures. Page 4. Comparison of Arteries, Capillaries, and Veins • Let's compare and contrast the three types of blood vessels: arteries, capillaries, and veins. • Label the artery, capillary and vein. Also label the layers of each. • Arteries are vessels that transport blood away from the heart. Because they are exposed to the highest pressures of any vessels, they have the thickest tunica media. -

Blood Vessels: Part A
Chapter 19 The Cardiovascular System: Blood Vessels: Part A Blood Vessels • Delivery system of dynamic structures that begins and ends at heart – Arteries: carry blood away from heart; oxygenated except for pulmonary circulation and umbilical vessels of fetus – Capillaries: contact tissue cells; directly serve cellular needs – Veins: carry blood toward heart Structure of Blood Vessel Walls • Lumen – Central blood-containing space • Three wall layers in arteries and veins – Tunica intima, tunica media, and tunica externa • Capillaries – Endothelium with sparse basal lamina Tunics • Tunica intima – Endothelium lines lumen of all vessels • Continuous with endocardium • Slick surface reduces friction – Subendothelial layer in vessels larger than 1 mm; connective tissue basement membrane Tunics • Tunica media – Smooth muscle and sheets of elastin – Sympathetic vasomotor nerve fibers control vasoconstriction and vasodilation of vessels • Influence blood flow and blood pressure Tunics • Tunica externa (tunica adventitia) – Collagen fibers protect and reinforce; anchor to surrounding structures – Contains nerve fibers, lymphatic vessels – Vasa vasorum of larger vessels nourishes external layer Blood Vessels • Vessels vary in length, diameter, wall thickness, tissue makeup • See figure 19.2 for interaction with lymphatic vessels Arterial System: Elastic Arteries • Large thick-walled arteries with elastin in all three tunics • Aorta and its major branches • Large lumen offers low resistance • Inactive in vasoconstriction • Act as pressure reservoirs—expand -

Blood and Lymph Vascular Systems
BLOOD AND LYMPH VASCULAR SYSTEMS BLOOD TRANSFUSIONS Objectives Functions of vessels Layers in vascular walls Classification of vessels Components of vascular walls Control of blood flow in microvasculature Variation in microvasculature Blood barriers Lymphatic system Introduction Multicellular Organisms Need 3 Mechanisms --------------------------------------------------------------- 1. Distribute oxygen, nutrients, and hormones CARDIOVASCULAR SYSTEM 2. Collect waste 3. Transport waste to excretory organs CARDIOVASCULAR SYSTEM Cardiovascular System Component function Heart - Produce blood pressure (systole) Elastic arteries - Conduct blood and maintain pressure during diastole Muscular arteries - Distribute blood, maintain pressure Arterioles - Peripheral resistance and distribute blood Capillaries - Exchange nutrients and waste Venules - Collect blood from capillaries (Edema) Veins - Transmit blood to large veins Reservoir Larger veins - receive lymph and return blood to Heart, blood reservoir Cardiovascular System Heart produces blood pressure (systole) ARTERIOLES – PERIPHERAL RESISTANCE Vessels are structurally adapted to physical and metabolic requirements. Vessels are structurally adapted to physical and metabolic requirements. Cardiovascular System Elastic arteries- conduct blood and maintain pressure during diastole Cardiovascular System Muscular Arteries - distribute blood, maintain pressure Arterioles - peripheral resistance and distribute blood Capillaries - exchange nutrients and waste Venules - collect blood from capillaries -

Arteries.Pdf
Arteries Although blood vessels differ in size, distribution, and function, structurally they share many common features. As in the heart, the walls of blood vessels consist of three major coats or tunics. Differences in the appearance and functions of the various parts of the circulatory system are reflected by structural changes in these tunics or by reduction and even omission of some of the layers. From the lumen outward, the wall of a blood vessel consists of a tunica intima, tunica media, and tunica adventitia. The tunica intima corresponds to and is continuous with the endocardium of the heart. It consists of an endothelium of flattened squamous cells resting on a basal lamina and is supported by a subendothelial connective tissue. The tunica media is the equivalent of the myocardium of the heart and is the layer most variable both in size and structure. Depending on the function of the vessel, this layer contains variable amounts of smooth muscle and elastic tissue. The tunica adventitia also varies in thickness in different parts of the vascular circuit. It consists mainly of collagenous connective tissue and corresponds to the epicardium of the heart, but it lacks mesothelial cells. As arteries course away from the heart they undergo successive divisions to provide numerous branches whose calibers progressively decrease. The changes in size and the corresponding changes in structure of the vessel wall are continuous, but three classes of arteries can be distinguished: large elastic or conducting arteries, medium-sized muscular or distributing arteries, and small arteries and arterioles. A characteristic feature of the entire arterial side of the blood vasculature system is the prominence of smooth muscle in the tunica media. -

Microscopic Anatomy of the Human Vertebro-Basilar System
MICROSCOPIC ANATOMY OF THE HUMAN VERTEBRO-BASILAR SYSTEM RENATO P. CHOPARD * — GUILHERME DE ARAÚJO LUCAS * ADRIANA LAUDANA* SUMMARY — Concerning the structure of connective-muscular components the authors stu died the walls of the terminal segments of the vertebral arteries as well as the basilar artery, utilizing the following staining methods: Azan modified by Heideinheim, Weigert's resor-¬ cin-fuchsin, and Weigert modified by van Gieson. It was established that wall of the verte-¬ bro-basilar system exhibits a mixed structure, muscular and elastic, by means of which the vessels are adjusted to the specific blood circulation conditions. Thus, vertebral arteries show in the most external layer of tunica media an evident external elastic lamina. In contrast, in the basilar artery the elastic tissue is localized mainly in the tunica media, and is distributed heterogeneously. In its caudal segment the elastic fibers are situated in the most internal layer of tunica media, and in the cranial segment the elastic component is homogenously distributed in the whole of tunica media. Anatomia microscópica do sistema, vértebro basilar no homem. RESUMO — Os autores estudam a disposição dos componentes fibromusculare» da parede das artérias vertebrais em seus segmentos terminais e a artéria basilar, usando os seguintes métodos de coloração: Azan modificado por Heidenheim, resorcina-fucsina de Weigert e Weigert modificado por van Gieson. O sistema vértebro-basilar apresentou em sua parede estrutura mista, muscular e elástica, pela qual os vasos se adaptam às condições circulató rias especificas daquela região. Besta forma, ias artérias vertebrais apresentam na camada mais externa da túnica média uma lâmina elástica limitante externa evidente, ao contrário do encontrado na artéria basilar. -

The Circulatory System
The Circulatory System Lec.10 Histology 1 The Circulatory System includes both 1. blood vascular system 2. lymphatic vascular system 2 blood vascular system 1. The heart, an organ whose function is to pump the blood. 2. The arteries, a series of efferent vessels that become smaller as they branch, and whose function is to carry the blood, with its nutrients and oxygen, to the tissues. 3. The capillaries, the smallest blood vessels, constituting a complex network of thin tubules that branch profusely in almost every organ and through whose walls the interchange between blood and tissues takes place. 4. The veins, which result from the convergence of capillaries into a system of larger channels that continue enlarging as they approach the heart, toward which they convey the blood to be pumped again. 3 The lymphatic vascular system • The lymphatic vascular system, begins with the lymphatic capillaries, which are closed-ended tubules that merge to form vessels of steadily increasing size; these vessels terminate in the blood vascular system emptying into the large veins near the heart. • One of the functions of the lymphatic system is to return the fluid of the tissue spaces to the blood. • The internal surface of all components of the blood and lymphatic systems is lined by a single layer of a squamous epithelium, called endothelium. 4 Heart • The heart is a muscular organ that contracts rhythmically, pumping the blood through the circulatory system . • The right and left ventricles pump blood to the lungs and the rest of the body respectively; right and left atria receive blood from the body and the pulmonary veins respectively. -
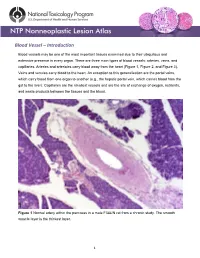
Blood Vessel – Introduction
Blood Vessel – Introduction Blood vessels may be one of the most important tissues examined due to their ubiquitous and extensive presence in every organ. There are three main types of blood vessels: arteries, veins, and capillaries. Arteries and arterioles carry blood away from the heart (Figure 1, Figure 2, and Figure 3). Veins and venules carry blood to the heart. An exception to this generalization are the portal veins, which carry blood from one organ to another (e.g., the hepatic portal vein, which carries blood from the gut to the liver). Capillaries are the smallest vessels and are the site of exchange of oxygen, nutrients, and waste products between the tissues and the blood. Figure 1 Normal artery within the pancreas in a male F344/N rat from a chronic study. The smooth muscle layer is the thickest layer. 1 Blood Vessel – Introduction Figure 2 Normal tunica media (asterisks) and tunica intima (arrows) of an artery within the pancreas in a male F344/N rat from a chronic study (higher magnification of Figure 1). Arteries and veins have walls composed of three layers: the tunica intima, the tunica media, and the tunica adventitia. The tunica intima is composed of endothelial cells on a basement membrane and a subendothelial layer of collagen and elastic fibers. The tunica media consists of smooth muscle cells, elastic fibers, and collagen. The tunica adventitia is composed of collagen and elastic fibers. The relative amounts of each of these components vary depending on the type of vessel. The tunica intima and tunica media of elastic arteries are generally thicker than those of the other types, and the tunica 2 Blood Vessel – Introduction Figure 3 Normal aorta in a male B6C3F1/N mouse from a chronic study. -

Special Histology and Embryology Cardiovascular System, Hematopoietic and Immune Protection System, Endocrine System 71
Special histology and embryology Cardiovascular system, Hematopoietic and immune protection system, endocrine system 71. In a red bone marrow specimen numerous capillaries are detected, through their wall mature blood elements enter blood circulation. What is the type of these capillaries? A. Lymphatic. B. Fenestrated. C. Somatic. D. Visceral. E. Sinusoidal. 72. Tunica intima of a vessel has been impregnated with argentic salt. As a result the cells with rugged, twisting edges have been detected. Name these cells. A. Stellate cells. B. Endotheliocytes. C. Myocytes. D.Fibroblasts. E. Adipocytes. 73. There are some tunics in the wall of blood vessels and heart. What tunic of the heart corresponds to a blood vessels wall according to its histogenesis and tissue structure? A. Myocardium. B. Endocardium. C. Pericardium. D.Epicardium. E. Epicardium and myocardium. 74. In a spleen specimen a blood ves--sel is detected. Its wall consists of basic membrane with endothelium, tunica media is absent, adventitia is grown together with connective tissue interlayers of spleen. What vessel is it? A. Arteriole. B. Vein of muscular type with poor development of muscular elements. C. Artery of muscular type. D.Vein of unmuscular type. E. Artery of elastic type. 75. Experimentally into the organism of a laboratory animal thymosin antibodies have been introduced. Differentiation of what cells will be affected first of all? A. T-lymphocytes. B. Monocytes. C. B-lymphocytes. D. Macrophages. E. Plasma cells. 76. In a histological specimen an organ parenchyma is represented by lym-phoid tissue which forms lymph nodules. These nodules are diffusely arranged and have a central artery. -

1. Arteries - Carry Blood Away from Heart
Anatomy Lecture Objectives Chapter 19 Chapter 19 - Vascular System A. categories and general functions: 1. arteries - carry blood away from heart 2. capillaries - allow exchange of materials between blood and tissue fluid 3. veins - return blood to heart B. wall structure - most blood vessel walls have 3 layers lumen = space inside vessel 1. tunica intima / tunica interna endothelium - simple squamous e. subendothelial layer - loose c.t. (collagen) 2. tunica media a. smooth muscle - cells circularly arranged controlled by ANS and chemical factors constriction (smooth muscle contracts) decreases blood flow and increases systemic blood pressure dilation (smooth muscle relaxes) increases blood flow and decreases systemic blood pressure b. elastic c.t. 3. tunica adventitia / tunica externa c.t. attaches vessel to surrounding structures vasa vasorum nourish outer part of vessel wall Strong/Fall 2008 page 1 Anatomy Lecture Objectives Chapter 19 C. arteries 1. elastic (conducting) - large arteries near heart (aorta and major branches) conduct blood to muscular arteries low resistance tunica media = circular elastin sheets with few smooth m. cells recoil maintains blood pressure during diastole 2. muscular - middle-sized arteries, distal to elastic arteries distal to elastic arteries tunica media very thick; much smooth m. and some elastin regulate blood flow to organs have an internal and an external elastic lamina 3. arterioles - smallest arteries tunica media contains smooth m. only diameter controlled by ANS and chemical messengers diameter determines blood flow and blood pressure D. capillaries wall consists of endothelium and basal lamina (no tunica media or externa) 8 to 10 mm in diameter join and branch to form capillary beds cells are joined at spots around perimeter by tight junctions and desmosomes intercellular clefts are spaces between cells 1.