New Data Оn Minerals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Downloaded for Personal Non-Commercial Research Or Study, Without Prior Permission Or Charge
MacArtney, Adrienne (2018) Atmosphere crust coupling and carbon sequestration on early Mars. PhD thesis. http://theses.gla.ac.uk/9006/ Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten:Theses http://theses.gla.ac.uk/ [email protected] ATMOSPHERE - CRUST COUPLING AND CARBON SEQUESTRATION ON EARLY MARS By Adrienne MacArtney B.Sc. (Honours) Geosciences, Open University, 2013. Submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy at the UNIVERSITY OF GLASGOW 2018 © Adrienne MacArtney All rights reserved. The author herby grants to the University of Glasgow permission to reproduce and redistribute publicly paper and electronic copies of this thesis document in whole or in any part in any medium now known or hereafter created. Signature of Author: 16th January 2018 Abstract Evidence exists for great volumes of water on early Mars. Liquid surface water requires a much denser atmosphere than modern Mars possesses, probably predominantly composed of CO2. Such significant volumes of CO2 and water in the presence of basalt should have produced vast concentrations of carbonate minerals, yet little carbonate has been discovered thus far. -

Orickite and Coyoteite, Two New Sulfide Minerals from Coyote Peak, Humboldt County, California
American Mineralogist, Volume 6E, pages 245-254, 1983 Orickite and coyoteite,two new sulfide minerals from Coyote Peak, Humboldt County, California Rrcneno C. Eno eNn Genall K. CzeuaNsrie U. S. Geological Survey Menlo Park, California 94025 Abstract Orickite and coyoteite occur with rare alkali iron sulfidesin a mafic alkalic diatreme near Orick, Humboldt County, California. Both minerals are very rare, and only a few milligrams of each have been found. Orickite,Na,KrCuse5Fer.ooSz.zHzO (x,y < 0.03,z < 0.5),is hexagonal;a:3.695, c : 6.164 (both t0.0lA); D = 4.212g cm-3 forZ:4. The six strongestlines in the X-ray diffraction powder pattern are [d in A, t, (*ttt):3.08, 100,(002); 3.20 90, (100);2.84,60, (l0l); 1.73,55, (103);1.583,30, (ll2);2.20, 15,(102). The mineralis brassyellow and opaque, weakly pleochroic, but strongly anisotropic (from grayish brown to grayish blue) in reflected light. Orickite is compositionally near iron-rich chalcopyrite, but the mineral may be related to synthetic chalcogenideshaving a distorted wurtzite-\2[I) structure. Coyoteite, NaFe3S5.2H2O,is triclinic, Pl or Pl; a : 7.409(8),b : 9.881(6),c = 6.441(3)A,a:100'25(3)',F=104"37(5)',y=81"29(5)':D=2.879ecm-3forZ=2.Thesix strongestlines in the powder patter! are: 5.12,100,(lll);7.13,90,(100);3.028,80,(220); 3.08O,70,(OO2);9.6,60,(010);5.60,60,(01l). Coyoteite is black and opaque;in reflectedlight it is pale brownish gray, faintly pleochroic, and strongly anisotropic (from gray to dull golden orange). -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

U.S. DEPARTMENT of the INTERIOR U.S.GEOLOGICAL SURVEY ALK.BIB a Selected Bibliography of Alkaline Igneous Rocks and Related Mine
U.S. DEPARTMENT OF THE INTERIOR U.S.GEOLOGICAL SURVEY ALK.BIB A selected bibliography of alkaline igneous rocks and related mineral deposits, with an emphasis on western North America compiled by Felix E. Mutschler, D. Chad Johnson, and Thomas C. Mooney Open-File Report 94-624 1994 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards and stratigraphic nomenclature. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. INTRODUCTION This bibliography contains 3,406 references on alkaline igneous rocks and related mineral deposits compiled in conjunction with ongoing studies of alkaline igneous rocks, metallogeny, and tectonics in western North America. Much of the literature on these topics is not readily recovered by searches of current bibliographies and computerized reference systems. We hope that by making this bibliography available, it will help other workers to access this occasionally hard to find literature. The bibliography is available in two formats: (1) paper hardcopy and (2) Apple Macintosh computer-readable 3.5 inch double density diskette. The computer-readable version of the bibliography is a 725 KB WORD (version 5.0) document. Individual literature citations are arranged alphabetically by author(s) and the order of items in each citation follows the standard U.S. Geological Survey format. Version 3.4 1 February 1994 BIBLIOGRAPHY Abbott, J. G., Gordey, S. P., and Tempelman-Kluit, D. J., 1986, Setting of stratiform, sediment- hosted lead-zinc deposits in Yukon and northeastern British Columbia, in Morin, J. -

IMA–CNMNC Approved Mineral Symbols
Mineralogical Magazine (2021), 85, 291–320 doi:10.1180/mgm.2021.43 Article IMA–CNMNC approved mineral symbols Laurence N. Warr* Institute of Geography and Geology, University of Greifswald, 17487 Greifswald, Germany Abstract Several text symbol lists for common rock-forming minerals have been published over the last 40 years, but no internationally agreed standard has yet been established. This contribution presents the first International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) approved collection of 5744 mineral name abbreviations by combining four methods of nomenclature based on the Kretz symbol approach. The collection incorporates 991 previously defined abbreviations for mineral groups and species and presents a further 4753 new symbols that cover all currently listed IMA minerals. Adopting IMA– CNMNC approved symbols is considered a necessary step in standardising abbreviations by employing a system compatible with that used for symbolising the chemical elements. Keywords: nomenclature, mineral names, symbols, abbreviations, groups, species, elements, IMA, CNMNC (Received 28 November 2020; accepted 14 May 2021; Accepted Manuscript published online: 18 May 2021; Associate Editor: Anthony R Kampf) Introduction used collection proposed by Whitney and Evans (2010). Despite the availability of recommended abbreviations for the commonly Using text symbols for abbreviating the scientific names of the studied mineral species, to date < 18% of mineral names recog- chemical elements -
Orickite and Coyoteite, Two New Sulfide Minerals from Coyote Peak, Humboldt County, California
American Mineralogist, Volume 68, pages 245-254, 1983 Orickite and coyoteite, two new sulfide minerals from Coyote Peak, Humboldt County, California RICHARD C. ERD AND GERALD K. CZAMANSKE U. S. Geological Survey Menlo Park, California 94025 Abstract Orickite and coyoteite occur with rare alkali iron sulfides in a mafic alkalic diatreme near Orick, Humboldt County, California. Both minerals are very rare, and only a few milligrams of each have been found. Orickite, NaxKyCuo.9sFe1.06Sz.zHzO (x,y < 0.03, z < 0.5), is hexagonal; a = 3.695, c = 6.16A (both :to.olA); D = 4.212 g cm-3 for Z = 4. The six strongest lines in the X-ray diffraction powder pattern are [d in A, I, (khl): 3.08, 100, (002); 3.20 90, (100); 2.84, 60, (101); 1.73, 55, (103); 1.583, 30, (112); 2.20, 15, (102). The mineral is brass yellow and opaque, weakly pleochroic, but strongly anisotropic (from grayish brown to grayish blue) in reflected light. Orickite is compositionally near iron-rich chalcopyrite, but the mineral may be related to synthetic chalcogenides having ajistorted wurtzite-(2H) structure. Coyoteite, NaFe3Ss'2HzO, is triclinic, PI or PI; a = 7.409(8), b = 9.881(6), c = 6.441(3)A, a = 100°25(3)', f3 = 104°37(5)', 81°29(5)'; D = 2.879 g cm-3 for Z = 2. The six 'Y = strongest lines in the powder patter~ are: 5.12,100,(111); 7.13,90,(100); 3.028,80,(220); 3.080,70,(002); 9.6,60,(010); 5.60,60,(011). -

1 Michael Fleischer and Mary Woodruff Open-File Report 88-689
U.S. DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY THE FORD-FLEISCHER FILE OF MINERALOGICAL REFERENCES, 1982-1987 INCLUSIVE by Michael Fleischer 1 and Mary Woodruff Open-File Report 88-689 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards. Dept. Mineral Sciences, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560 2 U.S. Geological Survey, MS 959, 12201 Sunrise Valley, Reston, VA 22092 1988 The Ford-Fleischer File of Mineralogical References 1982-1987 Inclusive In 1916, Prof. W.E. Ford of Yale University, having just published the Third Appendix to Dana's System of Mineralogy, 6th Edition, began to plan for the 7th Edition. He decided to create a file with a separate folder for each mineral (or for each mineral group) into which he would place a citation to any paper that contained data that should be considered in the revision of the 6th Edition. The file was maintained in duplicate with one copy going to Harvard University. In the early 1930's Palache, Berman, and Frondel at Harvard were designated chief revisionists. Assistants for the project included C.W. Wolfe and M.A. Peacock who were to gather crystallographic data at Harvard; and their counterpart, Michael Fleischer, who was to collect and evaluate chemical data at Yale. After Prof. Ford's death in March 1939, one set of his files came to the U.S. Geological Survey, and the literature has been covered since then by Michael Fleischer. Copies of the literature survey are maintained at the U.S. -

Appendix A: CK
Appendix A: App. Appendix Abbreviations and Symbols ark arkose AS sannaite (Fig.1.2; App.B) Explanation: As Association (Table 2.1) Chemical elements are referred to by assoc. associated with standard symbols (Ba, Ce, Cr, etc.). At actinolite Measurement units also have standard ban banatite symbols (em, ha, km, etc.) bas basalt Mineral names have two letters, starting Bi biotite-phlogopite with an initial capital letter (coined to By baryte avoid duplication with chemical element CA appinite (Fig.1.2; App.B) symbols). The only exceptions are Cpx CAL calc-alkaline lamp (App.B) and Opx, used because of their already Cb carbonate (Cc, Dl, etc.) widespread adoption. Normative cbt carbonarite minerals have the same abbreviations as Cc calcite modal minerals, but are italicised and in cc malchite (App.B) lower-case, e.g. ab, an, em, qz. CE kentallenite (Fig.1.2; App.B) Rock-names and other terms have more Ch chromite than two letters, all in lower case. C.I. colour index (modal mafics Lamprophyre varieties have 2/3 letters and Ci cordierite are in CAPITALS (e.g. AC), following CK kersantite (Fig.1.2; App.B) Fig.1.2, App.B. CM minette (Fig.l.2; App.B) Compound names are hyphenated e.g. Cm corundum grt-porph = granite-porphyry, Gt-gran cong conglomerate = garnet-granulite, Sp-lhz = spinel CP porphyry (App.B) lherzolite; they may include element Cpx clinopyroxene symbols, e.g.Cr-Di = chrome-diopside. cs spessartite (Fig.l.2; App.B) Ct chlorite Aa adularia cum cumulate Ab albite cv vogesite (Fig.l.2; App.B) AB bostonite (App.B) D.I. -

Metasomatism, Titanian Acmite, End Alkali Amphiboles in Lithic-Wacke
Amefican Mineralogist, Volwe 70, pages 499-516, 1985 Metasomatism,titanian acmite, end alkali amphibolesin lithic-wackeinclusions within the Coyote Peak diatreme,Humboldt County, California GsRAro K. Cz,c,MANsKEAND SrevEN A. ArrrNr U.S. Geological Suruey 345 Miilillefield Roail, Menlo Park, California 94025 Absfirct Lithic-wacke inclusions within the alkalic ultramafic diatreme at Coyote Peak record a remarkable history of metasomatismand crystal growth. Dominant metasomaticchanges were loss of Si from the inclusionsand massinflux of K, due to an unusuallyhigh K activity in the ultramafic host. Reactionwith K convertedmuch of the clay, quartz, and lithic fraction of the lithic wackeinto microclineand exchangedNa from abundantclastic albite. Sodium releasedfrom albite: (1) combined with Ti and Fe to form myriads of acrnite cystals, many of which are strongly zoned with cores unusually rich in titanium; (2) com- bined with Ti, Fe, Mg, and Ca to form alkali amphiboles,which are zoned from fluor- richterite cores to unusual titanian-arfvedsoniterims; and (3) formed a late-stagezeolite similar to natrolite. The sequenceof ferromagnesianphases is interpreted to have been controlled partly by falling temperature,but dominately by falling /o, as the oxidized sedi- mentaryassemblage equilibrated with the reducedultramafic melt. Preservationof primary sedimentarytextures to within a few millimeters of the lithic- wacke/ultramaficinterface in zoned inclusions and strong zonation betweenthe cores and rims of individual phasessuggest that recrystallizationand -

A Selective Lamprophyre Bibliography
A Selective Lamprophyre Bibliography Compilation This Bibliography includes most publications which: (a) contain the word lamprophyre (or one of the varieties named in Fig. 1.2 and Appendix B) either in the title or as a keyword; (b) devote significant attention to lamprophyres in terms of field, petrographical or petrological description; (c) contain analytical data on lamprophyres. Those in which lamprophyres are only very briefly mentioned, and sources prior to and including those compiled by Washington (1917), are not generally included unless giving information which is critical or otherwise unavailable. References to kimberlites are not exhaustively compiled - readers should refer for example to R.H.Mitchell (1986) or to the 7 International Kimberlite Conference (IKC) volumes. Abstracts and unpublished works are excluded, unless there is no other published information on the topic. This Bibliography has been compiled over a period of over 16 years by an exhaustive combination of traditional literature search (aided by Bibliography and Index of Geology, Bulletin Signa/etique, Dissertation Abstracts International and Mineralogical Abstracts), plus usage of the many computerized geological source and reference databases now available (specifically AESIS, GEOARCHIVES, GEOBASE, GEOREF and IGBA). Note that searches from these systems on the free-text strings "minette" and "spessartite" will also raise references to sedimentary rocks and to garnets (e.g. Siehl & Thein 1978). Available compilations dealing with alkaline rocks (e.g. Heinrich 1966; Tuttle & Gittins 1966; S(6rensen 1974; Fitton & Upton 1987; Woolley 1987; Bell 1989; Mem.Geol.SocJndial5) and the 7 IKC volumes have also been searched. The rate of acquisition has now slowed to the point where the Bibliography probably includes the vast majority of relevant references; coverage of Russian and Chinese publications is probably weakest. -
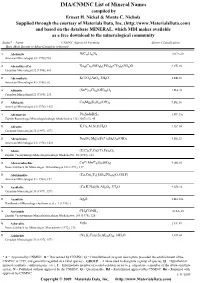
IMA/CNMNC List of Mineral Names
IMA/CNMNC List of Mineral Name s compiled by Ernest H. Nickel & Monte C. Nichols Supplied through the courtesy of Materials Data, Inc. (http://www.MaterialsData.com) and based on the database MINERAL, which MDI makes available as a free download to the mineralogical community Status* Name CNMNC Approved Formula Strunz Classification Best, Most Recent or Most Complete reference. A Abelsonite NiC£¡H£¢N¤ 10.CA.20 American Mineralogist 63 (1978) 930 A Abenakiite-(Ce) Na¢¦Ce¦(SiO£)¦(PO¤)¦(CO£)¦(SO¢)O 9.CK.10 Canadian Mineralogist 32 (1994), 843 G Abernathyite K(UO¢)AsO¤•3H¢O 8.EB.15 American Mineralogist 41 (1956), 82 A Abhurite (SnÀÈ)¢¡Cl¡¦(OH)¡¤O¦ 3.DA.30 Canadian Mineralogist 23 (1985), 233 D Abkhazite Ca¢Mg¥Si¨O¢¢(OH)¢ 9.DE.10 American Mineralogist 63 (1978), 1023 A Abramovite Pb¢SnInBiS§ 2.HF.25a Zapiski Rossiiskogo Mineralogicheskogo Obshchetstva 136 (2007) (5), 45 D Abrazite K,Ca,Al,Si,O,H¢O 9.GC.05 Canadian Mineralogist 35 (1997), 1571 D Abriachanite Na¢(Fe,Mg)£(FeÁÈ)¢Si¨O¢¢(OH)¢ 9.DE.25 American Mineralogist 63 (1978), 1023 D Absite (U,Ca,Y,Ce)(Ti,Fe)¢O¦ Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 92 (1963), 113 A Abswurmbachite CuÀÈ(MnÁÈ)¦O¨(SiO¤) 9.AG.05 Neues Jahrbuch für Mineralogie, Abhandlungen 163 (1991), 117 D Abukumalite (Ca,Ce)¢Y£(SiO¤,PO¤)£(O,OH,F) American Mineralogist 51 (1966), 152 D Acadialite (Ca,K,Na)(Si,Al)£O¦•3H¢O 9.GD.10 Canadian Mineralogist 35 (1997), 1571 G Acanthite Ag¢S 2.BA.30a Handbook of Mineralogy (Anthony et al.), 1 (1990), 1 A Acetamide CH£CONH¢ 10.AA.20 Zapiski Vsesoyuznogo Mineralogicheskogo