Summary of Investigation Results Itraconazole
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fungal Infections from Human and Animal Contact
Journal of Patient-Centered Research and Reviews Volume 4 Issue 2 Article 4 4-25-2017 Fungal Infections From Human and Animal Contact Dennis J. Baumgardner Follow this and additional works at: https://aurora.org/jpcrr Part of the Bacterial Infections and Mycoses Commons, Infectious Disease Commons, and the Skin and Connective Tissue Diseases Commons Recommended Citation Baumgardner DJ. Fungal infections from human and animal contact. J Patient Cent Res Rev. 2017;4:78-89. doi: 10.17294/2330-0698.1418 Published quarterly by Midwest-based health system Advocate Aurora Health and indexed in PubMed Central, the Journal of Patient-Centered Research and Reviews (JPCRR) is an open access, peer-reviewed medical journal focused on disseminating scholarly works devoted to improving patient-centered care practices, health outcomes, and the patient experience. REVIEW Fungal Infections From Human and Animal Contact Dennis J. Baumgardner, MD Aurora University of Wisconsin Medical Group, Aurora Health Care, Milwaukee, WI; Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, Madison, WI; Center for Urban Population Health, Milwaukee, WI Abstract Fungal infections in humans resulting from human or animal contact are relatively uncommon, but they include a significant proportion of dermatophyte infections. Some of the most commonly encountered diseases of the integument are dermatomycoses. Human or animal contact may be the source of all types of tinea infections, occasional candidal infections, and some other types of superficial or deep fungal infections. This narrative review focuses on the epidemiology, clinical features, diagnosis and treatment of anthropophilic dermatophyte infections primarily found in North America. -

Estimated Burden of Fungal Infections in Oman
Journal of Fungi Article Estimated Burden of Fungal Infections in Oman Abdullah M. S. Al-Hatmi 1,2,3,* , Mohammed A. Al-Shuhoumi 4 and David W. Denning 5 1 Department of microbiology, Natural & Medical Sciences Research Center, University of Nizwa, Nizwa 616, Oman 2 Department of microbiology, Centre of Expertise in Mycology Radboudumc/CWZ, 6500 Nijmegen, The Netherlands 3 Foundation of Atlas of Clinical Fungi, 1214GP Hilversum, The Netherlands 4 Ibri Hospital, Ministry of Health, Ibri 115, Oman; [email protected] 5 Manchester Fungal Infection Group, Manchester Academic Health Science Centre, The University of Manchester, Manchester M13 9PL, UK; [email protected] * Correspondence: [email protected]; Tel.: +968-25446328; Fax: +968-25446612 Abstract: For many years, fungi have emerged as significant and frequent opportunistic pathogens and nosocomial infections in many different populations at risk. Fungal infections include disease that varies from superficial to disseminated infections which are often fatal. No fungal disease is reportable in Oman. Many cases are admitted with underlying pathology, and fungal infection is often not documented. The burden of fungal infections in Oman is still unknown. Using disease frequencies from heterogeneous and robust data sources, we provide an estimation of the incidence and prevalence of Oman’s fungal diseases. An estimated 79,520 people in Oman are affected by a serious fungal infection each year, 1.7% of the population, not including fungal skin infections, chronic fungal rhinosinusitis or otitis externa. These figures are dominated by vaginal candidiasis, followed by allergic respiratory disease (fungal asthma). An estimated 244 patients develop invasive aspergillosis and at least 230 candidemia annually (5.4 and 5.0 per 100,000). -

Antifungals, Oral
Antifungals, Oral Therapeutic Class Review (TCR) July 13, 2018 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. July 2018 Proprietary Information. Restricted Access – Do not disseminate or copy without approval. © 2004-2018 Magellan Rx Management. All Rights Reserved. FDA-APPROVED INDICATIONS Drug Manufacturer FDA-Approved Indication(s) for oral use clotrimazole generic . -

Oral Antifungals Month/Year of Review: July 2015 Date of Last
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Class Update with New Drug Evaluation: Oral Antifungals Month/Year of Review: July 2015 Date of Last Review: March 2013 New Drug: isavuconazole (a.k.a. isavunconazonium sulfate) Brand Name (Manufacturer): Cresemba™ (Astellas Pharma US, Inc.) Current Status of PDL Class: See Appendix 1. Dossier Received: Yes1 Research Questions: Is there any new evidence of effectiveness or safety for oral antifungals since the last review that would change current PDL or prior authorization recommendations? Is there evidence of superior clinical cure rates or morbidity rates for invasive aspergillosis and invasive mucormycosis for isavuconazole over currently available oral antifungals? Is there evidence of superior safety or tolerability of isavuconazole over currently available oral antifungals? • Is there evidence of superior effectiveness or safety of isavuconazole for invasive aspergillosis and invasive mucormycosis in specific subpopulations? Conclusions: There is low level evidence that griseofulvin has lower mycological cure rates and higher relapse rates than terbinafine and itraconazole for adult 1 onychomycosis.2 There is high level evidence that terbinafine has more complete cure rates than itraconazole (55% vs. 26%) for adult onychomycosis caused by dermatophyte with similar discontinuation rates for both drugs.2 There is low -

Tinea Capitis 2014 L.C
BJD GUIDELINES British Journal of Dermatology British Association of Dermatologists’ guidelines for the management of tinea capitis 2014 L.C. Fuller,1 R.C. Barton,2 M.F. Mohd Mustapa,3 L.E. Proudfoot,4 S.P. Punjabi5 and E.M. Higgins6 1Department of Dermatology, Chelsea & Westminster Hospital, Fulham Road, London SW10 9NH, U.K. 2Department of Microbiology, Leeds General Infirmary, Leeds LS1 3EX, U.K. 3British Association of Dermatologists, Willan House, 4 Fitzroy Square, London W1T 5HQ, U.K. 4St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust, St Thomas’ Hospital, Westminster Bridge Road, London SE1 7EH, U.K. 5Department of Dermatology, Hammersmith Hospital, 150 Du Cane Road, London W12 0HS, U.K. 6Department of Dermatology, King’s College Hospital, Denmark Hill, London SE5 9RS, U.K. 1.0 Purpose and scope Correspondence Claire Fuller. The overall objective of this guideline is to provide up-to- E-mail: [email protected] date, evidence-based recommendations for the management of tinea capitis. This document aims to update and expand Accepted for publication on the previous guidelines by (i) offering an appraisal of 8 June 2014 all relevant literature since January 1999, focusing on any key developments; (ii) addressing important, practical clini- Funding sources cal questions relating to the primary guideline objective, i.e. None. accurate diagnosis and identification of cases; suitable treat- ment to minimize duration of disease, discomfort and scar- Conflicts of interest ring; and limiting spread among other members of the L.C.F. has received sponsorship to attend conferences from Almirall, Janssen and LEO Pharma (nonspecific); has acted as a consultant for Alliance Pharma (nonspe- community; (iii) providing guideline recommendations and, cific); and has legal representation for L’Oreal U.K. -

Therapies for Common Cutaneous Fungal Infections
MedicineToday 2014; 15(6): 35-47 PEER REVIEWED FEATURE 2 CPD POINTS Therapies for common cutaneous fungal infections KENG-EE THAI MB BS(Hons), BMedSci(Hons), FACD Key points A practical approach to the diagnosis and treatment of common fungal • Fungal infection should infections of the skin and hair is provided. Topical antifungal therapies always be in the differential are effective and usually used as first-line therapy, with oral antifungals diagnosis of any scaly rash. being saved for recalcitrant infections. Treatment should be for several • Topical antifungal agents are typically adequate treatment weeks at least. for simple tinea. • Oral antifungal therapy may inea and yeast infections are among the dermatophytoses (tinea) and yeast infections be required for extensive most common diagnoses found in general and their differential diagnoses and treatments disease, fungal folliculitis and practice and dermatology. Although are then discussed (Table). tinea involving the face, hair- antifungal therapies are effective in these bearing areas, palms and T infections, an accurate diagnosis is required to ANTIFUNGAL THERAPIES soles. avoid misuse of these or other topical agents. Topical antifungal preparations are the most • Tinea should be suspected if Furthermore, subsequent active prevention is commonly prescribed agents for dermatomy- there is unilateral hand just as important as the initial treatment of the coses, with systemic agents being used for dermatitis and rash on both fungal infection. complex, widespread tinea or when topical agents feet – ‘one hand and two feet’ This article provides a practical approach fail for tinea or yeast infections. The pharmacol- involvement. to antifungal therapy for common fungal infec- ogy of the systemic agents is discussed first here. -

Treatment Outcomes for Malassezia Folliculitis in the Dermatology Department of a University Hospital in Japan
Med. Mycol. J. Vol.Med. 57E, Mycol. E 63 J. − Vol. E 66, 57(No. 2016 3), 2016 E63 ISSN 2185 − 6486 Short Report Treatment Outcomes for Malassezia Folliculitis in the Dermatology Department of a University Hospital in Japan Chikako Suzuki, Midori Hase, Harunari Shimoyama and Yoshihiro Sei Department of Dermatology, Teikyo University Hospital ABSTRACT Topical or systemic antifungal therapy was administered to patients diagnosed with Malassezia folliculitis during the 5-year period between March 2007 and October 2013.The diagnosis of Malassezia folliculitis was established on the basis of characteristic clinical features and direct microscopic findings(10 or more yeast-like fungi per follicle).Treatment consisted of topical application of 2% ketoconazole cream or 100 mg oral itraconazole based on symptom severity and patientsʼ preferences. Treatment was given until papules flattened, and flat papules were examined to determine whether the patientʼs clinical condition had “improved” and the treatment had been “effective”.The subjects were 44 patients(35 men, 9 women), with a mean disease period of 25 ± 15 days.In regard to the lesion site, the frontal portion of the chest was the most common, accounting for 60% of all patients.The mean period required for improvement was 27 ± 16 days in 37 patients receiving the topical antifungal agent and 14 ± 4 days in the 7 patients receiving the systemic antifungal agent.The results were “improved” and the treatment was “effective” in all patients.Neither treatment resulted in any adverse reactions.Although administration of oral agents has been recommended for the treatment of Malassezia folliculitis, this study revealed that beneficial results are safely obtained with topical antifungal therapy alone, similar to those of systemic antifungal agents. -
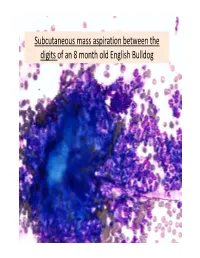
Subcutaneous Mass Aspiration Between the Digits of an 8 Month Old English Bulldog Marked Inflammation: Neutrophils & Macrophages
Subcutaneous mass aspiration between the digits of an 8 month old English Bulldog Marked inflammation: neutrophils & macrophages Hair fragment Macrophages What is this structure? Degenerate Neutrophils Abundant amount of keratin with similar structures The structures are ~ 2‐3 micron in size, blue to occasionally dark purple, oval to round in shape and have a thin clear capsule, consistent with fungal organisms The fungi are seen also in neutrophils and free in the background Growth of Microsporum canis was diagnosed on fungal culture • Microsporum canis is a dermatophyte that frequently causes infection of the superficial layers of skin, hair and nails (dermtophytoses). 1,2 • Microsporum canis, Microsporum gypseum and Trichophyton mentagrophytes are the main etiologic agents of clinical dermatophytosis. 3 • Dermatophytes affect the hair shafts, stratum corneum and nails or claws of animals and people. 2 • The typical lesions include focal alopecia, broken hair shafts, crusts scales and erythema on the head, feet and tail of dogs and cats. 1 • The structures seen in this cytology were Microsporum arthroconidia, which should be differentiated from other similar fungal yeast bodies seen in Candidiasis, Histoplasmosis, Aspergillosis/Penicillosis and Sporotrichosis. 2,4 Kerion • Less commonly dermatophytosis causes raised or dermal nodules called kerions or nodular dermatophytosis. 1,3 • These lesions are formed when the infected hair follicle ruptures and both the fungi and keratin spill in the dermis eliciting an intense inflammatory response. 1 • This may develop in any species but is most commonly reported in dogs. 3 • M. canis is the most common cause of kerion in dogs. 3 • Most common locations were the head, neck and limbs. -

Therapies for Common Cutaneous Fungal Infections
MedicineToday 2014; 15(6): 35-47 PEER REVIEWED FEATURE 2 CPD POINTS Therapies for common cutaneous fungal infections KENG-EE THAI MB BS(Hons), BMedSci(Hons), FACD Key points A practical approach to the diagnosis and treatment of common fungal • Fungal infection should infections of the skin and hair is provided. Topical antifungal therapies always be in the differential are effective and usually used as first-line therapy, with oral antifungals diagnosis of any scaly rash. being saved for recalcitrant infections. Treatment should be for several • Topical antifungal agents are typically adequate treatment weeks at least. for simple tinea. • Oral antifungal therapy may inea and yeast infections are among the dermatophytoses (tinea) and yeast infections be required for extensive most common diagnoses found in general and their differential diagnoses and treatments disease, fungal folliculitis and practice and dermatology. Although are then discussed (Table). tinea involving the face, hair- antifungal therapies are effective in these bearing areas, palms and T infections, an accurate diagnosis is required to ANTIFUNGAL THERAPIES soles. avoid misuse of these or other topical agents. Topical antifungal preparations are the most • Tinea should be suspected if Furthermore, subsequent active prevention is commonly prescribed agents for dermatomy- there is unilateral hand just as important as the initial treatment of the coses, with systemic agents being used for dermatitis and rash on both fungal infection. complex, widespread tinea or when topical agents feet – ‘one hand and two feet’ This article provides a practical approach fail for tinea or yeast infections. The pharmacol- involvement. to antifungal therapy for common fungal infec- ogy of the systemic agents is discussed first here. -

Canadian Clinical Practice Guideline on the Management of Acne (Full Guideline)
Appendix 4 (as supplied by the authors): Canadian Clinical Practice Guideline on the Management of Acne (full guideline) Asai, Y 1, Baibergenova A 2, Dutil M 3, Humphrey S 4, Hull P 5, Lynde C 6, Poulin Y 7, Shear N 8, Tan J 9, Toole J 10, Zip C 11 1. Assistant Professor, Queens University, Kingston, Ontario 2. Private practice, Markham, Ontario 3. Assistant Professor, University of Toronto, Toronto, Ontario 4. Clinical Assistant Professor, University of British Columbia, Vancouver, British Columbia 5. Professor, Dalhousie University, Halifax, Nova Scotia 6. Associate Professor, University of Toronto, Toronto, Ontario 7. Associate Clinical Professor, Laval University, Laval, Quebec 8. Professor, University of Toronto, Toronto, Ontario 9. Adjunct Professor, University of Western Ontario, Windsor, Ontario 10. Professor, University of Manitoba, Winnipeg, Manitoba 11. Clinical Associate Professor, University of Calgary, Calgary, Alberta Appendix to: Asai Y, Baibergenova A, Dutil M, et al. Management of acne: Canadian clinical practice guideline. CMAJ 2015. DOI:10.1503/cmaj.140665. Copyright © 2016 The Author(s) or their employer(s). To receive this resource in an accessible format, please contact us at [email protected]. Contents List of Tables and Figures ............................................................................................................. v I. Introduction ................................................................................................................................ 1 I.1 Is a Clinical Practice Guideline -

Seborrheic Dermatitis and Dandruff: a Comprehensive Review
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author J Clin Investig Manuscript Author Dermatol Manuscript Author . Author manuscript; available in PMC 2016 May 02. Published in final edited form as: J Clin Investig Dermatol. 2015 December ; 3(2): . Seborrheic Dermatitis and Dandruff: A Comprehensive Review Luis J. Borda and Tongyu C. Wikramanayake* Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, 1600 NW 10th Avenue, RMSB 2023A, Miami, Florida 33136, USA Abstract Seborrheic Dermatitis (SD) and dandruff are of a continuous spectrum of the same disease that affects the seborrheic areas of the body. Dandruff is restricted to the scalp, and involves itchy, flaking skin without visible inflammation. SD can affect the scalp as well as other seborrheic areas, and involves itchy and flaking or scaling skin, inflammation and pruritus. Various intrinsic and environmental factors, such as sebaceous secretions, skin surface fungal colonization, individual susceptibility, and interactions between these factors, all contribute to the pathogenesis of SD and dandruff. In this review, we summarize the current knowledge on SD and dandruff, including epidemiology, burden of disease, clinical presentations and diagnosis, treatment, genetic studies in humans and animal models, and predisposing factors. Genetic and biochemical studies and investigations in animal models provide further insight on the pathophysiology and strategies for better treatment. Keywords Seborrheic dermatitis; Dandruff; Sebaceous gland; Malassezia; Epidermal barrier Introduction Seborrheic Dermatitis (SD) and dandruff are common dermatological problems that affect the seborrheic areas of the body. They are considered the same basic condition sharing many features and responding to similar treatments, differing only in locality and severity. -

Fungal Infections (Mycoses): Dermatophytoses (Tinea, Ringworm)
Editorial | Journal of Gandaki Medical College-Nepal Fungal Infections (Mycoses): Dermatophytoses (Tinea, Ringworm) Reddy KR Professor & Head Microbiology Department Gandaki Medical College & Teaching Hospital, Pokhara, Nepal Medical Mycology, a study of fungal epidemiology, ecology, pathogenesis, diagnosis, prevention and treatment in human beings, is a newly recognized discipline of biomedical sciences, advancing rapidly. Earlier, the fungi were believed to be mere contaminants, commensals or nonpathogenic agents but now these are commonly recognized as medically relevant organisms causing potentially fatal diseases. The discipline of medical mycology attained recognition as an independent medical speciality in the world sciences in 1910 when French dermatologist Journal of Raymond Jacques Adrien Sabouraud (1864 - 1936) published his seminal treatise Les Teignes. This monumental work was a comprehensive account of most of then GANDAKI known dermatophytes, which is still being referred by the mycologists. Thus he MEDICAL referred as the “Father of Medical Mycology”. COLLEGE- has laid down the foundation of the field of Medical Mycology. He has been aptly There are significant developments in treatment modalities of fungal infections NEPAL antifungal agent available. Nystatin was discovered in 1951 and subsequently and we have achieved new prospects. However, till 1950s there was no specific (J-GMC-N) amphotericin B was introduced in 1957 and was sanctioned for treatment of human beings. In the 1970s, the field was dominated by the azole derivatives. J-GMC-N | Volume 10 | Issue 01 developed to treat fungal infections. By the end of the 20th century, the fungi have Now this is the most active field of interest, where potential drugs are being January-June 2017 been reported to be developing drug resistance, especially among yeasts.