Promotion of Research and Development in Drugs and Vaccines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SANJEEVAK 1586083 01/08/2007 MANOJ ANANT JOSHI Trading As ;AKSHAY PHARMA REMEDIES KAVRANA HOUSE, OPP
Trade Marks Journal No: 1836 , 12/02/2018 Class 5 SANJEEVAK 1586083 01/08/2007 MANOJ ANANT JOSHI trading as ;AKSHAY PHARMA REMEDIES KAVRANA HOUSE, OPP. COTTON GREEN RLY. STN. MUMBAI-400033. MANUFACTURE & MERCHANT INDIAN NATIONAL Used Since :31/01/2001 MUMBAI MEDICINAL PREPARATIONS. 537 Trade Marks Journal No: 1836 , 12/02/2018 Class 5 SEPTIGARD 1741718 08/10/2008 INDERJIT SINGH trading as ;INDERJIT SINGH B-104 , SWASTHYA SINGH , NEW DELHI -92 MERCHANTS & MANUFACTUERERS Address for service in India/Agents address: MAHTTA & CO. 43 - B/3, MAHTTA HOUSE,UDHAM SINGH NAGAR, LUDHIANA - 141 001, (PUNJAB). Proposed to be Used DELHI MEDICINAL & PHARMACEUTICAL PREPARATIONS. 538 Trade Marks Journal No: 1836 , 12/02/2018 Class 5 FAIR & BEAUTY 1803779 08/04/2009 GALPHA LABORATORIES LIMITED 221, Kanakia Zillion, E Wing Bandra Kurla Complex Annex LBS Marg & CST Road Junction Kurla West MUMBAI 400070 MANUFACTURERS AND MERCHANTS INDIAN NATIONAL Used Since :15/11/2007 MUMBAI PHARMACEUTICAL AND MEDICINAL PREPARATIONS AND SUBSTANCES 539 Trade Marks Journal No: 1836 , 12/02/2018 Class 5 O-BAMA 1815900 08/05/2009 KREMOINT PHARMA PVT. LTD. 151/5, SHRI KRISHNA DARSHAN, GARODIA NAGAR, GHATKOPAR (E), BOMBAY-400 077. MANUFACTURERS AND MERCHANTS. A CORPORATE ENTITY INCORPORATED IN INDIA UNDER THE COMPANIES ACT 1956. Address for service in India/Attorney address: KRISLAW CONSULTANTS BUILDING NO.4, C/104, SHANKESHWAR PALMS, BEHIND MODEL SCHOOL, KUMBHARKHANPADA, SUBHASH ROAD, DOMBIVILI(W) 421202 Used Since :02/05/2009 MUMBAI PHARMACETICAL AND MEDICINAL PREPARATIONS. 540 Trade Marks Journal No: 1836 , 12/02/2018 Class 5 AYUSHAKTI D-VYRO 1815928 08/05/2009 SMITA NARAM BUNGALOW NO.31, NEXT TO M. -
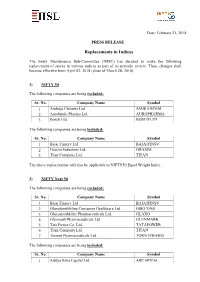
Replacements in Indices
Date: February 21, 2018 PRESS RELEASE Replacements in Indices The Index Maintenance Sub-Committee (IMSC) has decided to make the following replacement of stocks in various indices as part of its periodic review. These changes shall become effective from April 02, 2018 (close of March 28, 2018). 1) NIFTY 50 The following companies are being excluded: Sr. No. Company Name Symbol 1 Ambuja Cements Ltd. AMBUJACEM 2 Aurobindo Pharma Ltd. AUROPHARMA 3 Bosch Ltd. BOSCHLTD The following companies are being included: Sr. No. Company Name Symbol 1 Bajaj Finserv Ltd. BAJAJFINSV 2 Grasim Industries Ltd. GRASIM 3 Titan Company Ltd. TITAN The above replacements will also be applicable to NIFTY50 Equal Weight Index. 2) NIFTY Next 50 The following companies are being excluded: Sr. No. Company Name Symbol 1 Bajaj Finserv Ltd. BAJAJFINSV 2 GlaxoSmithkline Consumer Healthcare Ltd. GSKCONS 3 Glaxosmithkline Pharmaceuticals Ltd. GLAXO 4 Glenmark Pharmaceuticals Ltd. GLENMARK 5 Tata Power Co. Ltd. TATAPOWER 6 Titan Company Ltd. TITAN 7 Torrent Pharmaceuticals Ltd. TORNTPHARM The following companies are being included: Sr. No. Company Name Symbol 1 Aditya Birla Capital Ltd. ABCAPITAL Sr. No. Company Name Symbol 2 Ambuja Cements Ltd. AMBUJACEM 3 Aurobindo Pharma Ltd. AUROPHARMA 4 Bosch Ltd. BOSCHLTD 5 General Insurance Corporation of India GICRE 6 L&T Finance Holdings Ltd. L&TFH 7 SBI Life Insurance Company Ltd. SBILIFE 3) NIFTY 500 The following companies are being excluded: Sr. No. Company Name Symbol 1 Adani Enterprises Ltd. ADANIENT 2 Ahluwalia Contracts (India) Ltd. AHLUCONT 3 Apar Industries Ltd. APARINDS 4 AstraZenca Pharma India Ltd. ASTRAZEN 5 Corporation Bank CORPBANK 6 Dalmia Bharat Ltd. -

50 KW Offer Solar Copy 2
21st Century Enviro Engineers Pvt.Ltd. Plot No. 120(10 Marla), COMPANY Industrial Area Phase II Chandigarh - 160002, India PROFILE www.21stcenturyenviro.com INTRODUCTION 21st CENTURY ENVIRO ENGINEERS PVT. LTD. is a Company dealing in the field of Environmental Engineering related activities. We are Registered Environmental Consultants of Pollution Control Board to Supply ETP’s, STP’s, APCD’s, Incinerators, Water Treatment Plants, Reverse Osmosis, Evaporators, Solid Waste Management and Rain Water Harvesting and to carry out EIA Studies . The Directors of the company are young Technocrats having versatile experience in this field. We have a back up of highly qualified and experienced technical team having versatile experience in Designing, Erection, Commissioning and Operation of different types of Effluent Treatment Plants. Our Managing Director himself is a Chemical Engineer with almost 25 Years Experience in this line and Technical Director is PhD. in Environmental Science with almost 30 Years working experience on various types of effluent treatment Technologies. Our team comprises of almost 150 people from different backgrounds e.g. Chemical, Environmental, Mechanical, Instrumentation, Civil, Accounts, Purchase, Marketing etc. We have our Marketing/ Design Office in Chandigarh and regional offices in Dhaka, Mumbai, New Delhi, Sikkim etc. and have our own testing Laboratory for Effluent/Water Testing and to study treatability of effluent on pilot scale. We have our own full-fledged manufacturing unit of Pollution Control Equipments in Village Kunjhal Baddi (HP). We also undertake annual operation and maintenance contracts and liasining services for the systems supplied by us. We are also Registered with CREST (Chandigarh Renewable Energy & Technology Promotion Society) and SECI (Solar Energy Corporation of India) for supplying Online/Offline Solar systems in Chandigarh, Himachal Pradesh, Uttarakhand, J&K, Punjab, Haryana, Delhi, Bihar etc. -

Investor Presentation May 2016
Investor Presentation May 2016 BSE: 532523 │ NSE: BIOCON │ REUTERS: BION.NS │ BLOOMBERG: BIOS IN │ WWW.BIOCON.COM Safe Harbor Certain statements in this release concerning our future growth prospects are forward-looking statements, which are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those contemplated in such forward-looking statements. Important factors that could cause actual results to differ materially from our expectations include, amongst others general economic and business conditions in India, our ability to successfully implement our strategy, our research and development efforts, our growth and expansion plans and technological changes, changes in the value of the Rupee and other currencies, changes in the Indian and international interest rates, change in laws and regulations that apply to the Indian and global biotechnology and pharmaceuticals industries, increasing competition in and the conditions of the Indian biotechnology and pharmaceuticals industries, changes in political conditions in India and changes in the foreign exchange control regulations in India. Neither the company, nor its directors and any of the affiliates have any obligation to update or otherwise revise any statements reflecting circumstances arising after this date or to reflect the occurrence of underlying events, even if the underlying assumptions do not come to fruition. 2 Agenda Biocon: Who are we? Highlights • Business Highlights • Financial Highlights Growth Segments • -

Index Stock Update >> Marico Stock Update >> Gabriel India Stock Update >> PTC India Stock
Visit us at www.sharekhan.com November 04, 2015 Index Stock Update >> Marico Stock Update >> Gabriel India Stock Update >> PTC India Stock Update >> Ipca Laboratories Stock Update >> Skipper Viewpoint >> Lloyd Electric & Engineering For Private Circulation only REGISTRATION DETAILS Regd Add: Sharekhan Limited, 10th Floor, Beta Building, Lodha iThink Techno Campus, Off. JVLR, Opp. Kanjurmarg Railway Station, Kanjurmarg (East), Mumbai – 400042, Maharashtra. Tel: 022 - 61150000. Sharekhan Ltd.: SEBI Regn. Nos. BSE - INB/INF011073351 ; BSE- CD ; NSE - INB/ INF231073330 ; CD-INE231073330 ; MSEI - INB/INF261073333 ; CD-INE261073330 ; DP - NSDL-IN-DP-NSDL-233-2003 ; CDSL-IN-DP-CDSL-271-2004 ; PMS- INP000000662 ; Mutual Fund-ARN 20669 ; Commodity trading through Sharekhan Commodities Pvt. Ltd.: MCX-10080 ; (MCX/TCM/CORP/0425) ; NCDEX- 00132 ; (NCDEX/TCM/CORP/0142) ; NCDEX SPOT-NCDEXSPOT/116/CO/11/20626 ; For any complaints email at [email protected] ; Disclaimer: Client should read the Risk Disclosure Document issued by SEBI & relevant exchanges and Do’s & Don’ts by MCX & NCDEX and the T & C on www.sharekhan.com before investing. investor’s eye stock update Marico Reco: Buy Stock Update Enhanced focus to improve volume growth; maintain Buy CMP: Rs399 Company details Key points Price target: Rs460 Mixed operating performance: During Q2FY2016, Marico’s revenue grew by 4% to Rs1,485.4 crore, entirely driven by a 4% volume growth (domestic business’ volume Market cap: Rs25,735 cr growth stood at 5.5%). The gross profit margin (GPM) improved by almost 500BPS to 52 week high/low: Rs466/299 49.3% on the back of ~30% decline in the copra prices and 33% decline in the prices of liquid paraffin. -

Hy Sun Missed the Pharma Rally: the Answer Is Hidden in a Bet Many Failed — Speciality Drugs - the Economic Times
12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets News Industry RISE Politics Wealth MF Tech Jobs Opinion NRI Panache ET NOW More Aayush English Edition | E-Paper Tech Consumer Markets Corporate Governance Telecom + OTT Auto + Aviation Pharma Fintech + BFSI Economy Infra Environment Energy Business News › Prime › Pharma › Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Getty Images MARKETS hy Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Dilip Shanghvi, founder and managing director, Sun Pharmaceuticals Synopsis Sun Pharma is the only Indian company to have made some inroads into speciality drugs. What worries investors is high investments and uncertainties over ramp up in revenue. The stock can still see upside because of its current valuations, strong India business, and any positive surprises in US generic business. But it is crucial that its speciality bet pays off. BACK TO TOP https://economictimes.indiatimes.com/prime/pharma-and-healthcare/why-sun-missed-the-pharma-rally-the-answer-is-hidden-in-a-bet-many-failed-spe… 1/11 12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets NeCwasllI nitd uas trbyleRssISiEngP oilniti cdsisWgeuailthse fMoFr tTheceh InJodbisanOpinion NRI Panache ET NOW More pharmaceutical industry. The stocks of pharma companies have been on a tear since the beginning of the Covid-19 crisis. -

CIPLA, Ltd. CIPLA Ltd Is a Pharmaceutical Company Based in India
CIPLA Ltd. And the Provision of Anti-AIDS Pharmaceuticals It’s 2001, and G. G. Brereton is a busy man. He heads the Global Intellectual Property Office at GlaxoSmithKline (GSK), a multinational pharmaceutical corporation with operations throughout the world. He is tasked with defending GSK’s patents against foreign imitators. In recent months he has been occupied with infringements against GSK patents for antiretroviral drugs – the pharmaceuticals used to treat the symptoms of HIV and AIDS. This week has been especially hectic: CIPLA Ltd. of India has just announced its offer to supply an antiretroviral “cocktail” to Sub-Saharan African countries for 3 percent of the price that GSK charges for its anti-AIDS cocktail. Top management at GSK wants options for responding to this surprising offer. G. G. has been given the job of (1) coming up with potential responses by GSK to this initiative and (2) identifying that option that best assures the continued profitability of the firm now and into the foreseeable future. He has till Friday to do it! HIV/AIDS. One of the most vexing public-health problems of the new century has been the explosion in cases of human immunodeficiency virus (HIV) and the acquired immunodeficiency syndrome (AIDS). In the year 2000, the United Nation AIDS Organization (UNAIDS) reported that 36.1 million people worldwide were living with HIV or AIDS. There were 5.3 million new cases of HIV infection in the year, and 3.0 million deaths attributable to HIV infection. Since its first diagnosis, the AIDS epidemic has claimed 21.8 million lives. -

Pharmaceuticals Stellar Quarter; Healthy Outlook Sector Update
Pharmaceuticals Stellar quarter; Healthy Outlook Sector Update Q2FY2021 was yet another stellar quarter for pharmaceutical companies under Q2FY2021 Results Review Sharekhan’s pharma universe. The quarter witnessed a sustained improvement in Sector: Pharmaceuticals the US business while the domestic business showed signs of improvement. The overall plant utilization levels during Q2FY2021 were at normal levels (as against Sector View: Positive relatively low utilization levels in previous quarter), thus yielding costs benefits. For the quarter, the pharma companies delivered revenue growth of 8.8% y-o-y, while sequentially as well it clocked a growth of 8.3%. Topline performance was better than estimates and was driven by sturdy performance of the US business, while the India operations also grew a decent pace. Growth in the base business, stabilizing price erosion and new launches led to the growth in US business, while in India business, companies with a relatively higher share of chronic business grew strongly and outperformed the industry. Select players such as Aurobindo, Cadila and Cipla reported strong 12.5%, 18% and 10% y-o-y growth respectively in the US business. Divis labs also reported a strong 21% y-o-y growth in the topline backed by a strong performance in the API segment, while Laurus labs posted a 60% revenue Our active coverage universe growth on the back of an impressive growth in the formulations segment. Operating CMP PT profit for the universe increased sharply by 21% y-o-y. Operating margin also Companies Reco. (Rs) (Rs) expanded by 251 bps y-o-y, better the expected 150 bps y-o-y expansion. -

Cipla Ltd (CIPLA)
Cipla Ltd (CIPLA) CMP: | 826 Target: | 975 (18%) Target Period: 12 months BUY January 30, 2021 Strong growth in India, RoW; better margins… Q3 revenues grew 18.2% YoY to | 5169 crore led by strong growth in domestic and RoW markets. Domestic sales grew 25.5% YoY to | 2231 crore. RoW markets business grew 47.2% YoY to | 823 whereas South Particulars Africa business fell 2.5% YoY to | 579 crore. US grew 9.6% YoY to | 1037 crore. EBITDA margins expanded 647 bps YoY to 23.8% mainly due to P articular Am ount significantly lower other expenditure. Hence, EBITDA grew 62.3% YoY to | Market Capitalisation | 66581 crore 1231 crore. PAT more than doubled to | 748 crore vs. | 351 crore in Q3FY20. Debt (FY20) | 2816 crore Cash (FY20) | 1004 crore Result Update Result Product launches, front-end shift key for formulation exports E V | 68393 crore 52 week H/L (|) 870/357 Formulation exports comprise ~54% of FY20 revenues. The company is E quity capital | 161.3 crore focusing on front-end model, especially for the US, along with a gradual shift from loss making HIV and other tenders to more lucrative respiratory and F ace value | 2 Price performance other opportunities in the US and EU. We expect export formulation sales to grow at 9.8% CAGR to | 12242 crore in FY20-23E. Key drivers will be a launch of inhalers (drug-device) and other products in developed markets. 1000 14000 12000 800 Indian formulations growth backed by new launches 10000 600 8000 With ~5% market share, Cipla is the third largest player in the domestic 400 6000 formulations market. -

Pharmaceutical Cluster in Andhra Pradesh
Pharmaceutical Cluster in Andhra Pradesh Microeconomics of Competitiveness Final Project Harvard Business School Helene Herve | Lhakpa Bhuti | Saurabh Agarwal | Sonny Kushwaha | Akbar Causer May 2013 Table of Contents 1 Executive Summary ............................................................................................................................ 3 2 Introduction to India ........................................................................................................................... 4 2.1 History and Political Climate ....................................................................................................... 5 2.2 Competitive Positioning of India ................................................................................................. 6 2.2.1 Endowments .......................................................................................................................... 6 2.2.2 Economic Performance To-Date and Macroeconomic Policy .............................................. 7 2.2.3 Summary of Export Clusters ................................................................................................. 9 2.2.4 Social Infrastructure and Political Institutions .................................................................... 10 2.2.5 India Diamond .................................................................................................................... 11 3 Andhra Pradesh ................................................................................................................................ -

Domestic Institutional Philanthropy in India
Charting a course post COVID-19 Supported by Delivering High Impact SEP 2020 Published by Sattva in September 2020. Supported by the Bill & Melinda Gates Foundation. Email [email protected] Website www.sattva.co.in Lead Researchers Aarti Mohan, Sanjana Govil, Bhavin P Chhaya Research, Analysis Prachur Goel, Shalini Rajan, and Production Mansi Agarwal, Palagati Lekhya Reddy, Ashwini Rajkumar, Radhika Bose, Gayatri Malhotra, Brooke VanSant, Anushka Bhansali Project Advisors Karuna Segal, Arnav Kapur, Anjani Kumar Singh (Bill & Melinda Gates Foundation), Srikrishna Sridhar Murthy (Sattva Consulting) Design and Bhakthi Dakshinamurthy Typesetting [email protected] Cover Photo Axis Bank Foundation We are grateful to 74 leaders representing 47 organisations in the domestic institutional philanthropy ecosystem who generously shared their expertise and insights for this report (See Annexure 3 for a complete List of Interviews). This work is licensed under the Attribution-Non Commercial-ShareALike 4.0 International License: Attribution - You may give appropriate credit, provide a link to the license, and indicate if any changes were made. NonCommercial - You may not use the material for commercial purposes. ShareALike - If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original. About this study 01 Introduction 04 Executive summary 06 The landscape before and after COVID-19 08 Trends in intervention strategies 15 Trends in programme implementation 20 Programme trends per sector 27 Agricultural livelihoods Water and sanitation Women’s empowerment and digital financial inclusion Health systems and delivery Approaches to scale and collaboration 36 Annexures 41 Explanation of terms Study scope and methodology List of interviews Endnotes About 51 Dr. -
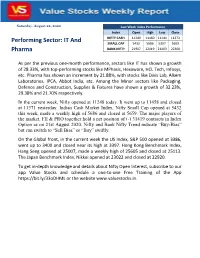
Nifty Poised to Open Gap up on Strong Payroll Data
Saturday, August 22, 2020 Last Week Index Performance Index Open High Low Close NIFTY CASH 11249 11460 11144 11372 Performing Sector: IT And SMALL CAP 5432 5686 5357 5659 Pharma BANK NIFTY 21907 22419 21403 22300 As per the previous one-month performance, sectors like IT has shown a growth of 28.33%, with top-performing stocks like MPhasis, Hexaware, HCL Tech, Infosys, etc. Pharma has shown an increment by 21.88%, with stocks like Divis Lab, Alkem Laboratories, IPCA, Abbot India, etc. Among the Minor sectors like Packaging, Defence and Construction, Supplies & Fixtures have shown a growth of 32.23%, 29.38% and 21.70% respectively. In the current week, Nifty opened at 11248 today. It went up to 11458 and closed at 11371 yesterday. Indian Cash Market Index, Nifty Small Cap opened at 5432 this week, made a weekly high of 5686 and closed at 5659. The major players of the market, FII & PRO together hold a net position of (-) 31419 contracts in Index Option as on 21st August 2020. Nifty and Bank Nifty Trend indicate “Buy-Bias” but can switch to “Sell Bias” or “Buy” swiftly. On the Global front, in the current week the US Index, S&P 500 opened at 3386, went up to 3400 and closed near its high at 3397. Hong Kong Benchmark Index, Hang Seng opened at 25007, made a weekly high of 25605 and closed at 25113. The Japan Benchmark Index, Nikkei opened at 23022 and closed at 22920. To get in-depth knowledge and details about Nifty Open Interest, subscribe to our app Value Stocks and schedule a one-to-one Free Training of the App https://bit.ly/33oDHML or the website www.valuestocks.in.