Year in Review 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Industrial Context Work Plan
LOS ANGELES CITYWIDE HISTORIC CONTEXT STATEMENT Context: Industrial Development, 1850-1980 Prepared for: City of Los Angeles Department of City Planning Office of Historic Resources September 2011; rev. February 2018 The activity which is the subject of this historic context statement has been financed in part with Federal funds from the National Park Service, Department of the Interior, through the California Office of Historic Preservation. However, the contents and opinions do not necessarily reflect the views or policies of the Department of the Interior or the California Office of Historic Preservation, nor does mention of trade names or commercial products constitute endorsement or recommendation by the Department of the Interior or the California Office of Historic Preservation. This program receives Federal financial assistance for identification and protection of historic properties. Under Title VI of the Civil Rights Act of 1964, Section 504 of the Rehabilitation Act of 1973, and the Age Discrimination Act of 1975, as amended, the U.S. Department of the Interior prohibits discrimination on the basis of race, color, national origin, disability, or age in its federally assisted programs. If you believe you have been discriminated against in any program, activity, or facility as described above, or if you desire further information, please write to: Office of Equal Opportunity, National Park Service; 1849 C Street, N.W.; Washington, D.C. 20240 SurveyLA Citywide Historic Context Statement Industrial Development, 1850-1980 TABLE -

Complimentary Facial Promotion
WIN with the Website! Our team is excited to announce that we've launched a new website and we want you to have an opportunity to WIN! 1) Visit CRMerleNorman.com and look at the spa services. 2) Find the "WIN with the website" image on our social pages: Facebook: Campbell River Merle Norman Facebook: Something More Aesthetics Instagram @CRMerleGirl Instagram @SomethingMoreAesthetics 3) Like the post and comment with which service you'd like the On Monday, February 15th we will opportunity to win. Each comment counts as an entry for a chance randomly select a winner! to WIN! Good luck! Complimentary Facial Promotion We are half way through our Complimentary Facial Promotion and it has been a huge success - Thank you! If you'd like to participate, this is how. Bring home 3 of your favorite Eminence Organic Skincare products and enjoy a complimentary 70 minute organic facial (value of $110) or 3 of your favorite Merle Norman Skincare products and enjoy a complimentary 45 minute facial (value of $85). Eminence bonus: participate in the promotion and get an entry for a chance to win a 90 minute Eminence Pedicure (value of $75). Draw date February 27th. Merle Norman bonus: participate in the promotion get a complimentary gift with purchase (while supplies last) Questions? Call us 250.286.0622 or email here facial inquiry Valentine's Day Inspiration Treat your loved one (or hint to them to treat you), to a gift certificate for a spa experience this Valentine’s Day. Visit our studio for an extensive collection of organic skincare, cosmetics, jewelry, nail polish, purses, scarves, bath products, essential oils, accessories and Valentine’s baskets. -

Most Influential
CUSTOM CONTENT OCTOBER 28, 2019 MOST INFLUENTIAL FAMILY-OWNED BUSINESSES Academic Achievers • Accredited Home Health Services • The Albright • AMCAL • AMS Fulfillment • The Annenberg Foundation • Art Lewin Bespoke Clothiers BADMAASH • Baron Equities, Inc. Beachwood Market • Berman, Berman, Berman, Schneider & Lowary, LLP • Bobbi-Toads C C R Properties, Inc. California Receivership Group • Century Precision Engineering Inc. • The Chanani Group/Bluestem Hotel Consumer Law Experts, PC • Couture Salon & Spa • Decron Properties • Divine Dips Dulan's Soul Food • Etco Homes F. Gavina & Sons, Inc. • Feldmar Watch Co. Inc. Felikian’s Carpet One • Frazier Aviation, Inc. Freight Right Global Logistics • Galpin Motors The Garland • GEARYS Beverly Hills • Gelt, Inc. Golden Star Technology Inc. • Goldrich Kest Hawthorne Advertising • Jeff & Tony's DSD Kings Arch • LBW Insurance and Financial Services Majestic Realty Co. • MATT Construction Merle Norman Cosmetics • Milt & Edie’s Dry Cleaners & Tailoring Center • Montage Insurance Solutions The MSM Technology Group • Nathan Kimmel Company, LLC NorthStar Moving Company • Pacific American Fish Company Nourmand & Associates P2s Inc. • Paul’s Photo • Ramonas Food Group LLC/ Ramonas Restaurant Group LLC • RealTax • RiseUp Technologies • Rolling Robots • RP & Associates • S. Bravo Systems, Inc. • Sage Millimeter Shepherd’s Door Domestic Violence Resource Center • Smog City Brewing Co • SMS Transportation Services Inc. • Someone’s in the Kitchen • Stepp Commercial Swatfame, Inc. • Talley, Inc. • Torrance Bakery • Turner Techtronics, Inc. • Vromage • WESSCO International • World Oil Corp. • ZipTire Mobile Tire Sales and Installation 056-80_FOB.indd 56 10/24/19 1:43 PM OCTOBER 28, 2019 CUSTOM CONTENT – LOS ANGELES BUSINESS JOURNAL 57 MOST INFLUENTIAL FAMILY-OWNED BUSINESSES Letter from the Publisher ccording to the U.S. -

Year in Review 2018 Personal Care Products Council Year in Review 2018
YEAR IN REVIEW 2018 PERSONAL CARE PRODUCTS COUNCIL YEAR IN REVIEW 2018 TABLE OF CONTENTS A MESSAGE FROM THE PRESIDENT & CEO 1 2 3 4 For the Personal Care Products Council (PCPC), 2018 These efforts are a prelude to what will be a very MESSAGE ASSOCIATION MESSAGE MODERNIZING FROM AT A GLANCE FROM GOVERNMENT was a year of significant achievement. It was a year exciting 2019 as we prepare to celebrate PCPC's 125th PRESIDENT BOARD CHAIR POLICIES that saw vigorous debate in the public policy arena anniversary. Our industry contributes to the nations' & CEO as our industry worked with leading policymakers health and well-being and plays a critical role in the and advocacy groups to modernize state and federal U.S. economy. We support millions of domestic jobs, laws while working to ensure a fair and realistic invest billions in research and development to drive business environment for cosmetics and personal innovation, and generate taxes to support services care companies. and programs in the communities in which our companies operate. PCPC member companies manufacture, distribute 7 8 11 and supply the vast majority of products marketed in Looking ahead, our industry remains steadfast in its ABOUT THE PROMOTING DRIVING the U.S. Product safety, grounded in the best science commitment to a healthy future, one that continues to PERSONAL SOUND SCIENCE GLOBAL ACCESS CARE PRODUCTS available, remained a top priority for our industry be grounded in the best science available while also INDUSTRY in 2018. addressing sustainability priorities in all aspects of our businesses. Together, with the millions of families who To drive global access for our members’ products, depend on our products, we look forward to a shared PCPC worked to align domestic and international future that helps to create a more beautiful world in regulatory practices and eliminate trade barriers for all its forms. -

Happy Mother's
OUR LADY OF HOPE CATHOLIC CHURCH 4675 S. CLYDE MORRIS BLVD. , PORT ORANGE, FL 32129 Mailing address: P.O. Box 290216, Port Orange, L 32129 Office 788-6144 EMERGENCY (after hours) 386-846-5761 Office Hours: Monday-Friday 9:00am-4:00pm closed noon-1pm Happy Mother’s Day to all Mothers May God Bless Each of You On your special day and throughout the year! Sunday, May 9, 2021 Sixth Sunday of Easter This is my commandment; love one another as I love you. John 15:12 MASS SCHEDULE: Daily Mass 8:30am Monday-Friday WEEKEND MASSES: Vigil 4:00pm; Sunday 7:30am; 9:00am & 11:00am Sunday Mass is on our website: ladyofhope.org STAFF Business Manager RCIA Coordinator Pastor Vincent LaVigna 788-6144, Ext. 306 Jasmine Rosales788-6144 Father Matthew Mello E-mail: [email protected] E-mail: [email protected] 788-6144, Ext. 311 Operations Manager Thrift Store E-Mail: [email protected] Maureen Kealhofer 788-6144 Ext 304 Chris Ahlswede –767-0571 Parochial Vicar E-Mail: [email protected] Father Titus “Fr. Kachy” Kachinda Facilities Manager Deacon Michael Pettit, R. N. CCM 788-6144, Ext. 310 Jerry MacKenzie 788-6144, Ext. 318 386-679-7256 E:Mail: [email protected] E-Mail: [email protected] E-Mail: [email protected] Director of Liturgy\Minister of Music Manager Altar Server Coordinator/Young Adult Funeral Coordinator Fr. Raymond J. O’Leary Parish Center Deacon Mike Rosolino 316-0128 Daniel V. Weimer 788-6144, ext. 314 John Rodgers 788-6144 E-Mail:[email protected] E-Mail: [email protected] Minister of Youth Deacon Mike Willems 386-299-4490 Evangelization and Discipleship [email protected] Administrative Coordinator/Secretary Rowena Hoblin 788-6747 ~~~~~~~~~~~~~~~~~~~~~~~~~~~ Chiquita Rodgers 788-6144, ext 301 E-mail: faithformation@ladyof hope.org Website: ladyofhope.org E-Mail: [email protected] Facebook: #OLOHPortOrange Welcome to everyone in attendance today! Whether you are traveling from across the street or country, thank you for choosing to spend part of your weekend with our Christian community of believers. -

FRANCHISE DISCLOSURE DOCUMENT Merle Norman Cosmetics, Inc
FRANCHISE DISCLOSURE DOCUMENT Merle Norman Cosmetics, Inc. A California Corporation 9130 Bellanca Avenue Los Angeles, California 90045 (310) 337-2200 www.merlenorman.com [email protected] The Studio Owner will operate a retail store known as a “Studio,” which sells Merle Norman cosmetic products. The total investment necessary to begin operation of a Merle Norman Cosmetic Studio ranges from approximately $91,891 to $190,778 for a Studio located in a regional mall and from approximately $61,891 to $125,778 for a Studio that is not located in a regional mall and uses standard New Design fixtures and from $51,936 to $95,649 for a Studio that is not located in a regional mall and uses E-Design fixtures. This includes the price for one of several initial packages of Merle Norman Cosmetics, supplies and other items which range from approximately $13,000 to $24,000 that must be paid to Merle Norman. The total investment does not include rent for the business location. This Disclosure Document summarizes certain provisions of your franchise agreement and other information in plain English. Read this Disclosure Document and all accompanying agreements carefully. You must receive this Disclosure Document at least 14 calendar-days before you sign a binding agreement with, or make any payment to, the franchisor or an affiliate in connection with the proposed franchise sale. Note, however, that no governmental agency has verified the information contained in this document. The terms of your contract will govern your franchise relationship. Don’t rely on the Disclosure Document alone to understand your contract. -

The World's Most Active Cosmetics Professionals on Social - August 2021
The World's Most Active Cosmetics Professionals on Social - August 2021 Industry at a glance: Why should you care? So, where does your company rank? Position Company Name LinkedIn URL Location Employees on LinkedIn No. Employees Shared (Last 30 Days) % Shared (Last 30 Days) 1 Wella Company https://www.linkedin.com/company/wella-company/Switzerland 2,167 256) 11.81% 2 La Prairie https://www.linkedin.com/company/la-prairie-group/Switzerland 984 107) 10.87% 3 Rituals https://www.linkedin.com/company/rituals/Netherlands 3,289 309) 9.39% 4 Davines https://www.linkedin.com/company/davines/Italy 705 64) 9.08% 5 Laboratoires Filorga https://www.linkedin.com/company/laboratoires-filorga/France 520 47) 9.04% 6 Nuxe https://www.linkedin.com/company/nuxe/France 709 64) 9.03% 7 Laboratoires Expanscience https://www.linkedin.com/company/laboratoires-expanscience/France 975 85) 8.72% 8 Molton Brown https://www.linkedin.com/company/molton-brown/United Kingdom 541 47) 8.69% 9 Naos https://www.linkedin.com/company/naos-bioderma---institut-esthederm---etat-pur-/France 2,220 175) 7.88% 10 Charlotte Tilbury https://www.linkedin.com/company/charlotte-tilbury-beauty-ltd/United Kingdom 1,140 89) 7.81% 11 Caudalie https://www.linkedin.com/company/caudalie/France 818 62) 7.58% 12 Aesop https://www.linkedin.com/company/aesop/Australia 1,331 96) 7.21% 13 Sesderma https://www.linkedin.com/company/laboratorios-sesderma/Spain 506 35) 6.92% 14 Guerlain https://www.linkedin.com/company/guerlain/France 2,113 144) 6.81% 15 Beiersdorf https://www.linkedin.com/company/beiersdorf/Germany -

CTB 55+ Club Participating Merchant Discount Book November 4, 2020
CTB 55± Club Participating Merchant Discount Book Ask your local Community Trust Banker or visit us online to find out more about the EQUAL HOUSING LENDE R Member FDIC CTB 55+ Club Checking Account Revised 11/04/2020 https://www.ctbi.com/ctbi/personal-banking/personal-checking Table of Contents Below you will find cities and towns listed under their county or a neighboring county. You can then turn to the page with that county’s listings to find the merchant you are looking for. If you can’t find your favorite merchant, ask them to call your local CTB 55+ Club Coordinator (see page 3) to sign up as a participating merchant. Central Eastern Northeast South Central Region Region Region Region pp. 4-7 pp. 8-10 pp. 11-12 pp. 13-18 Boyle County Floyd County Boyd County Adair County Danville Ivel Ashland Columbia Lackey Clark County Minnie Fleming County Bell County Winchester Prestonsburg Flemingsburg Middlesboro Paintsville Maysville Pineville Fayette County Louisa Washington (And surrounding areas) Green County Lexington Letcher County Greenup County Greensburg Nicholasville Jenkins Flatwoods Neon Knox-Laurel-Whitley Franklin County Whitesburg Nicholas County, WV Counties Frankfort Craigsville London Mingo County, WV Richwood Corbin Madison County Delbarton Summersville Williamsburg Berea Williamson Richmond Marion County Pike County Lebanon Mercer County Belfry Harrodsburg Breaks, VA Pulaski County Coal Run Somerset Montgomery County Dorton Mt. Sterling Elkhorn City Taylor County Forest Hills Campbellsville Woodford County Johns Creek Versailles Mouthcard Rockcastle County Myra Mt. Vernon Phyllis Pikeville Russell County Regina Jamestown Robinson Creek Tug Valley Anderson County, TN Virgie Clinton Campbell County, TN LaFollette Jacksboro CTB 55± Club Coordinators Below are the names and contact information of our CTB 55+ Club Coordinators and the counties they service. -

The World's Most Active Cosmetics Professionals on Social
The USA's Most Active Cosmetics Professionals on Social - August 2021 Industry at a glance: Why should you care? So, where does your company rank? Position Company Name LinkedIn URL Location Employees on LinkedIn No. Employees Shared (Last 30 Days) % Shared (Last 30 Days) 1 Voyant Beauty https://www.linkedin.com/company/voyantbeauty/United States 640 30) 4.69% 2 The Estée Lauder https://www.linkedin.com/company/the-estee-lauder-companies-inc/United States 25,784 1,115) 4.32% 3 JPMS https://www.linkedin.com/company/john-paul-mitchell-systems/United States 631 23) 3.65% 4 Farmasi https://www.linkedin.com/company/farmasi/United States 4,409 157) 3.56% 5 bareMinerals https://www.linkedin.com/company/bareminerals/United States 1,460 45) 3.08% 6 Benefit Cosmetics https://www.linkedin.com/company/benefitcosmetics/United States 3,849 100) 2.60% 7 Revlon https://www.linkedin.com/company/revloninc/United States 5,944 136) 2.29% 8 Jafra https://www.linkedin.com/company/jafra-cosmetics/United States 3,031 58) 1.91% 9 Mary Kay https://www.linkedin.com/company/marykayglobal/United States 55,175 1,001) 1.81% 10 Aveda https://www.linkedin.com/company/aveda/United States 3,201 58) 1.81% 11 Elizabeth Arden https://www.linkedin.com/company/elizabeth-arden/United States 1,874 31) 1.65% 12 Bobbi Brown Cosmetics https://www.linkedin.com/company/bobbi-brown-cosmetics/United States 1,637 27) 1.65% 13 Wella https://www.linkedin.com/company/wella/United States 1,098 17) 1.55% 14 MAC https://www.linkedin.com/company/mac-cosmetics/United States 11,927 135) 1.13% 15 Merle Norman Cosmetics https://www.linkedin.com/company/merle-norman-cosmetics/United States 1,069 11) 1.03% 16 Clinique https://www.linkedin.com/company/clinique/United States 6,822 65) 0.95% 17 Boots Retail USA https://www.linkedin.com/company/no7beautycompanyusa/United States 1,266 6) 0.47% 18 Beauty Systems https://www.linkedin.com/company/beauty-systems-group/United States 792 1) 0.13% Ready to win at social? Set up takes just 6 minutes and you can try free for 30-days, no credit card needed and you cancel at any time. -
Mulberry Grove
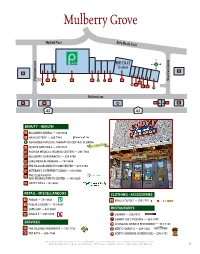
Mulberry Grove Mayfield Place Belle Meade Circle S W E N 21 20 Pebblebrook Court 2 3 4 5 6 7 8 9 Moss Creek Avenue 12 1 10 11 13 14 15 Mulberry Lane 19 18 24 22 23 16 BEAUTY • HEALTH 2 MULBERRY DENTAL — 753-0784 3 HAIR CUTTERY — 205-7441 6 ADVANCED PHYSICAL THERAPY OF CENTRAL FLORIDA 8 JQ HAIR AND NAILS — 259-2727 13 FLORIDA MEDICAL HEARING CENTERS — 205-7804 14 MULBERRY CHIROPRACTIC — 259-4708 18 LAKE MEDICAL IMAGING — 787-5858 19 THE VILLAGES HEALTH CARE CENTER — 674-1750 20 VETERAN’S OUTPATIENT CLINIC — 674-5000 21 THE CLUB HEALTH AND REHABILITATION CENTER — 385-8200 22 HAPPY NAILS – 751-6453 RETAIL - MISCELLANEOUS CLOTHING • ACCESSORIES 4 PUBLIX — 751-0301 9 BEALLS OUTLET — 259-7781 5 PUBLIX LIQUORS — 753-4541 12 SAFE SHIP — 847-0848 RESTAURANTS 16 CIRCLE K — 205-8796 1 SUBWAY — 750-9991 7 SAMMY JOE’S PIZZERIA — 633-1949 SERVICES 10 SHANGHAI CHINESE RESTAURANT — 751-1133 11 THE VILLAGES INSURANCE — 753-7755 15 BEEF O’ BRADY’S — 259-1536 23 PEP BOYS — 205-7746 24 CODY’S ORIGINAL ROADHOUSE — 205-7797 Business listings and locations shown are current as of MAY 2019 but are subject to change without notice or any liability whatsoever between reprints. © Holding Company of The Villages, Inc., 2019, All Rights Reserved. The Villages is a registered trademark of Holding Company of The Villages, Inc. 5 Spanish Plaines El Camino Real S W N E Banderos Avenue 18 1 2 3 Buenos Aires Boulevard 9 10 11 12 13 19 4 5 8 6 7 Bella Cruz Drive Bella Cruz Drive 14 15 27 441 16 27 17 441 BEAUTY • HEALTH MISCELLANEOUS 1 BETTER AT HOME — 753-9119 2 WHOLE -

View Complaint
CaseCase 2:11-cv-00404-MHS 2:12-cv-00539-MHS Document Document 10 2 Filed 11/03/1108/28/12 Page 1 of 27 PageID #: 745 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION GEOTAG INC. AND GEOTAG, INC., Civil Action No. 2:11-CV-404 Plaintiffs, v. JURY TRIAL DEMANDED EYE CARE CENTERS OF AMERICA INC., AMERICAN GREETINGS CORPORATION, HALLMARK CARDS, INC., HICKORY FARMS INC., PAPYRUS FRANCHISE CORPORATION, SPENCER GIFTS LLC, INTERNATIONAL COFFEE & TEA, LLC D/B/A THE COFFEE BEAN & TEA LEAF, THINGS REMEMBERED, INC. THE YANKEE CANDLE COMPANY, INC., BOSE CORP., GUITAR CENTER INC., PROGRESSIVE CONCEPTS INC., RITZ CAMERA IMAGE LLC, 24 HOUR FITNESS WORLDWIDE INC., BALLY TOTAL FITNESS CORP., BARE ESCENTUALS INC., BIOSCRIP INC., CRABTREE & EVELYN, CURVES INTERNATIONAL, INC., GOLD’S GYM INTERNATIONAL INC., GREAT CLIPS INC., L.A. FITNESS INTERNATIONAL LLC, LIFE TIME FITNESS INC., M.A.C. COSMETICS, INC. MEDICINE SHOPPE INTERNATIONAL INC., MERLE NORMAN COSMETICS, VITAMIN COTTAGE NATURAL FOOD MARKETS D/B/A NATURAL GROCERS BY VITAMIN COTTAGE, REGIS CORPORATION, SALLY BEAUTY SUPPLY LLC, SEPHORA USA INC., THE BODY SHOP AMERICAS, INC., CaseCase 2:11-cv-00404-MHS 2:12-cv-00539-MHS Document Document 10 2 Filed 11/03/1108/28/12 Page 2 of 27 PageID #: 756 TONI&GUY USA, LLC, ULTA SALON, COSMETICS & FRAGRANCE, INC., VITAMIN SHOPPE INDUSTRIES, INC., VIVA VITAMINS, EYEMART EXPRESS, LTD., LUXOTTICA RETAIL NORTH AMERICA INC., NATIONAL VISION INC., U.S. VISION INC., PET SUPPLIES PLUS-USA INC.,AND WILD BIRDS UNLIMITED INC., Defendants. PLAINTIFFS’ FIRST AMENDED COMPLAINT This is an action for patent infringement in which Plaintiffs GeoTag Inc. -

Shopping Center & Retail Guide
Shopping Center & Retail Guide 2016-2017 Edition Shopping Center & Retail Guide Spectrum Marketplace 1 Massage Green Spa Weight Watchers A to Z Hollistic Center Serenity MD Amazing Lash Studio Lavender Care Day Spa Northwest corner of Grand Av. & Pipeline Av., near 71 Fwy. Hear USA Broker: Chris Walton, Newmark (949) 608-2096 Chino Promenade Retailers Rancho del Chino 3 5 The Avenue Northside of Philadelphia St. between Central Av. & Benson Av. Spectrum Towne Center Chino Southeast corner of Ramona Av. & Eucalyptus Av. Bath and Body Works 2 Broker: Terry Bortnick, Argent Retail Advisors Brokers: Jared Himes and Lidia Talavera, NAI Capital Commercial (888) 301-1888 Progressive RE, (949) 344-1200 and (909) 945-2339 Cigarettes USA Southwest corner of Grand Av. & Pipeline Av., near 71 Fwy. Curling Iron Salon II & Supplies Broker: Dan Samulski, CBRE, Retailers (949) 725-8595 Retailers Dick’s Sporting Goods Espirit Nail Spa Bella Spa Edible Arrangements Retailers Home Depot Chino Nails Fashion Q AT&T JC Penny Cuts 4 Kids Melody Nail & Spa Best Buy Solus Clothing Game ER Men’s Wearhouse Big 5 Sporting Goods Sephora (inside JC Penny) GNC Merle Norman Cosmetics Carter’s Restaurants International Hair Design Michael’s Crafts Catherine’s Plus Sizes McDonald’s Lollie’s Wig Shop Motherhood Maternity Famous Footwear Papachino’s Grill & Greens Movies 8 Theatre Old Navy Game Stop Canabru Coffee Smoke’N Mart Ortho Mattress Happy Nails Auraganic St. Catherine’s Vintage & Treasures Pep Boys Lane Bryant Services Sweet Tooth Alley PETCO Kohl’s Agape Fitness Bootcamp Restaurants Photo Solutions Kut N Beauty Scottrade Financial Services Great China Super Buffet Rejuvenation Spa II Marshalls Yoga Body Hans BBQ Teriyaki Ross Dress for Less Massage Envy Advantage Real Estate Juan Pollo Sears Home Appliance Showroom Music n Smart OroMar Silkscreen Pho Lotus Staples Nordstrom Rack Maiden Wellnes Pizza Hut T.J.