Spatial Packing, Cranial Base Angulation, and Craniofacial Shape
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nasal Morphology and Its Correlation to Craniofacial Morphology in Lateral Cephalometric Analysis
International Journal of Environmental Research and Public Health Article Nasal Morphology and Its Correlation to Craniofacial Morphology in Lateral Cephalometric Analysis Agnieszka Jankowska 1 , Joanna Janiszewska-Olszowska 2,* and Katarzyna Grocholewicz 2 1 Private Practice “Dental Clinic Jankowscy”, 68-200 Zary,˙ Poland; [email protected] 2 Department of Interdisciplinary Dentistry, Pomeranian Medical University in Szczecin, 70-111 Szczecin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-91-466-1690 Abstract: Nose shape, size, and inclination influence facial appearance, but few studies concern the relationship between the nasal profile and craniofacial structures. The objective of this study was to analyze association of nasal cephalometric variables with skeletal structures, age, and sex. Cephalometric and nasal analysis was performed in 386 Polish orthodontic patients (aged 9–25 years). Student t-test and Mann–Whitney test were used to compare quantitative variables and Pearson’s or Spearman’s correlation coefficients—to find correlations. Soft tissue facial convexity angle corre- lates to Holdaway ratio, ANB (A-Nasion-B), and Wits appraisal. Nasal dorsum axis, nose length, nose depth (1) and nose depth (2), nose hump, lower dorsum convexity, and columella convexity increase with age. Nasal base angle, nasolabial angle, nasomental angle, soft tissue facial convex- ity and nasal bone angle decrease with age. Nasal base angle and nasomental angle are smaller in females. Thus, a relationship exists between nasal morphology and sagittal jaw configuration. Nasal parameters significantly change with age. Sexual dimorphism characterizes nasal bone angle Citation: Jankowska, A.; and nasomental angle. Janiszewska-Olszowska, J.; Grocholewicz, K. Nasal Morphology Keywords: nose; nose profile; cephalometry; orthodontics and Its Correlation to Craniofacial Morphology in Lateral Cephalometric Analysis. -
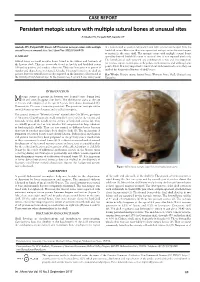
Persistent Metopic Suture with Multiple Sutural Bones at Unusual Sites
CASE REPORT Persistent metopic suture with multiple sutural bones at unusual sites Ambade HV, Fulpatil MP, Kasote AP Ambade HV, Fulpatil MP, Kasote AP. Persistent metopic suture with multiple in a human skull at asterion, left pterion and right coronal suture apart from the sutural bones at unusual sites. Int J Anat Var. 2017;10(3):69-70. lambdoid suture. Moreover, there was a persistent metopic suture between bregma to nasion in the same skull. The metopic suture with multiple sutural bones SUMMARY spreading beyond lambdoid suture at unusual sites is not reported previously. The knowledge of such variation and combination is rare and very important Sutural bones are small irregular bones found in the sutures and fontanels of for forensic expert, radiologists, orthopedists, neurosurgeons and anthropologist the human skull. They are commonly found at lambda and lambdoid suture point of view. It is very important to know about such variation because they can followed by pterion; and rarely at other sites. They vary from person to person in mislead the diagnosis of fracture of skull bones. number and shape, hence not named. Usually, 1-3 sutural bones in one skull are present, but 8-10 sutural bones are also reported in the literature, all restricted in Key Words: Metopic suture; Sutural bones; Wormian bones; Skull; Unusual sites; the vicinity of lambdoid sutures. In the present case, 8 sutural bones were present Variations INTRODUCTION etopic suture is present in between two frontal bones during fetal Mlife and soon disappear after birth. The obliteration starts at the age of 2 years and completed at the age of 8 years from above downwards (1). -

Cranial-Base Morphology in Children with Class Iii Malocclusion
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Cranial-base morphology in Class III malocclusion CRANIAL-BASE MORPHOLOGY IN CHILDREN WITH CLASS III MALOCCLUSION Hong-Po Chang, Shu-Hui Hsieh,1 Yu-Chuan Tseng,2 and Tsau-Mau Chou Faculty of Dentistry and 1Graduate Institute of Dental Sciences, Kaohsiung Medical University, and 2Department of Orthodontics, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan. The association between cranial-base morphology and Class III malocclusion is not fully understood. The purpose of this study was to investigate the morphologic characteristics of the cranial base in children with Class III malocclusion. Lateral cephalograms from 100 children with Class III malocclusion were compared with those from 100 subjects with normal occlusion. Ten landmarks on the cranial base were identified and digitized. Cephalometric assessment using seven angular and 18 linear measurements was performed by univariate and multivariate analyses. The results revealed that the greatest between-group differences occurred in the posterior cranial-base region. It was concluded that shortening and angular bending of the cranial base, and a diminished angle between the cranial base and mandibular ramus, may lead to Class III malocclusion associated with Class III facial morphology. The association between cranial- base morphology and other types of malocclusion needs clarification. Further study of regional changes in the cranial base, with geometric morphometric analysis, is warranted. Key Words: cephalometric analysis, children, Class III malocclusion, cranial base, morphology (Kaohsiung J Med Sci 2005;21:159–65) A Class III malocclusion, defined by the mandibular first attempts were made to characterize global craniofacial permanent molar being “mesial” (i.e. -

Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Identifying the Misshapen Head: Craniosynostosis and Related Disorders Mark S. Dias, MD, FAAP, FAANS,a Thomas Samson, MD, FAAP,b Elias B. Rizk, MD, FAAP, FAANS,a Lance S. Governale, MD, FAAP, FAANS,c Joan T. Richtsmeier, PhD,d SECTION ON NEUROLOGIC SURGERY, SECTION ON PLASTIC AND RECONSTRUCTIVE SURGERY Pediatric care providers, pediatricians, pediatric subspecialty physicians, and abstract other health care providers should be able to recognize children with abnormal head shapes that occur as a result of both synostotic and aSection of Pediatric Neurosurgery, Department of Neurosurgery and deformational processes. The purpose of this clinical report is to review the bDivision of Plastic Surgery, Department of Surgery, College of characteristic head shape changes, as well as secondary craniofacial Medicine and dDepartment of Anthropology, College of the Liberal Arts characteristics, that occur in the setting of the various primary and Huck Institutes of the Life Sciences, Pennsylvania State University, State College, Pennsylvania; and cLillian S. Wells Department of craniosynostoses and deformations. As an introduction, the physiology and Neurosurgery, College of Medicine, University of Florida, Gainesville, genetics of skull growth as well as the pathophysiology underlying Florida craniosynostosis are reviewed. This is followed by a description of each type of Clinical reports from the American Academy of Pediatrics benefit from primary craniosynostosis (metopic, unicoronal, bicoronal, sagittal, lambdoid, expertise and resources of liaisons and internal (AAP) and external reviewers. However, clinical reports from the American Academy of and frontosphenoidal) and their resultant head shape changes, with an Pediatrics may not reflect the views of the liaisons or the emphasis on differentiating conditions that require surgical correction from organizations or government agencies that they represent. -

Morphometry of the Midfacial Complex in Subjects with Class III Malocclusions: Procrustes, Euclidean, and Cephalometric Analyses
Clinical Anatomy 11:162–170 (1998) Morphometry of the Midfacial Complex in Subjects With Class III Malocclusions: Procrustes, Euclidean, and Cephalometric Analyses G.D. SINGH,1* J.A. MCNAMARA, JR.,2 AND S. LOZANOFF3 1Department of Dental Surgery and Periodontology, Dundee Dental Hospital and School, University of Dundee, Dundee, Scotland, UK 2Department of Orthodontics and Pediatric Dentistry, School of Dentistry and Center for Human Growth and Development, University of Michigan, Ann Arbor, Michigan 3Departments of Anatomy and Reproductive Biology and of Surgery, John A. Burns School of Medicine, University of Hawaii, Honolulu, Hawaii The purpose of this study was to determine whether the morphology of the midface differed in subjects with a retrognathic midfacial appearance (Class III malocclusions) using a combina- tion of morphometric and cephalometric analyses. After obtaining appropriate consent, lateral cephalographs of 133 children of European-American descent, ages 5–11 years, were compared: 73 had Class III malocclusion, 60 had normal (Class I) occlusion. The cephalo- graphs were traced and subdivided into seven age- and sex-matched groups. Average geometries based upon seven nodes (pterygoid point, PTS; rhinion, RO; posterior nasal spine, PNS; midpalatal point, MPP; anterior nasal spine, ANS; subspinale, A; prosthion, Pr), scaled to an equivalent size, were compared using a Procrustes routine. Euclidean distance matrix analysis (EDMA) was employed to localize differences in morphology. Bivariate analyses on unscaled data utilizing nine linear and six angular measurements were also undertaken. Results from Procrustes and EDMA analyses indicated that although the overall midfacial configura- tions differed statistically (P , 0.05), only about half of the seven age sub-groups maintained significance. -

Level I to III Craniofacial Approaches Based on Barrow Classification For
Neurosurg Focus 30 (5):E5, 2011 Level I to III craniofacial approaches based on Barrow classification for treatment of skull base meningiomas: surgical technique, microsurgical anatomy, and case illustrations EMEL AVCı, M.D.,1 ERINÇ AKTÜRE, M.D.,1 HAKAN SEÇKIN, M.D., PH.D.,1 KUTLUAY ULUÇ, M.D.,1 ANDREW M. BAUER, M.D.,1 YUSUF IZCI, M.D.,1 JACQUes J. MORCOS, M.D.,2 AND MUSTAFA K. BAşKAYA, M.D.1 1Department of Neurological Surgery, University of Wisconsin–Madison, Wisconsin; and 2Department of Neurological Surgery, University of Miami, Florida Object. Although craniofacial approaches to the midline skull base have been defined and surgical results have been published, clear descriptions of these complex approaches in a step-wise manner are lacking. The objective of this study is to demonstrate the surgical technique of craniofacial approaches based on Barrow classification (Levels I–III) and to study the microsurgical anatomy pertinent to these complex craniofacial approaches. Methods. Ten adult cadaveric heads perfused with colored silicone and 24 dry human skulls were used to study the microsurgical anatomy and to demonstrate craniofacial approaches in a step-wise manner. In addition to cadaveric studies, case illustrations of anterior skull base meningiomas were presented to demonstrate the clinical application of the first 3 (Levels I–III) approaches. Results. Cadaveric head dissection was performed in 10 heads using craniofacial approaches. Ethmoid and sphe- noid sinuses, cribriform plate, orbit, planum sphenoidale, clivus, sellar, and parasellar regions were shown at Levels I, II, and III. In 24 human dry skulls (48 sides), a supraorbital notch (85.4%) was observed more frequently than the supraorbital foramen (14.6%). -

1 TERMINOLOGIA ANTHROPOLOGICA Names of The
TERMINOLOGIA ANTHROPOLOGICA Names of the parts of the human body, terms of aspects and relationships, and osteological terminology are as in Terminologia Anatomica. GENERAL TERMS EXPLANANTION ADAPTATION Adjustment and change of an organism to a specific environment, due primarily to natural selection. ADAPTIVE RADIATION Divergence of an ancestral population through adaption and speciation into a number of ecological niches. ADULT Fully developed and mature individual ANAGENESIS The progressive adaption of a single evolutionary line, where the population becomes increasingly specialized to a niche that has remained fairly constant through time. ANCESTRY One’s family or ethnic descent, the evolutionary or genetic line of descent of an animal or plant / Ancestral descent or lineage ANTEMORTEM Biological processes that can result in skeletal modifications before death ANTHROPOCENTRICISM The belief that humans are the most important elements in the universe. ANTHROPOLOGY The study of human biology and behavior in the present and in the past ANTHROPOLOGIST BIOLOGICAL A specialist in the subfield of anthropology that studies humans as a biological species FORENSIC A specialist in the use of anatomical structures and physical characteristics to identify a subject for legal purposes PHYSICAL A specialist in the subfield of anthropology dealing with evolutionary changes in the human bodily structure and the classification of modern races 1 SOCIAL A specialist in the subfield of anthropology that deals with cultural and social phenomena such as kingship systems or beliefs ANTHROPOMETRY The study of human body measurement for use in anthropological classification and comparison ARCHETYPE That which is taken as the blueprint for a species or higher taxonomic category ARTIFACT remains of past human activity. -
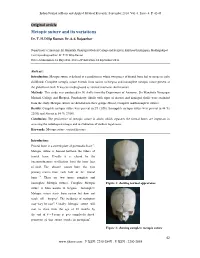
Metopic Suture and Its Variations Dr
Indian Journal of Basic and Applied Medical Research; September 2014: Vol.-3, Issue- 4, P. 42-45 Original article Metopic suture and its variations Dr. T. H. Dilip Kumar, Dr. S. S. Rajasekar Department of Anatomy, Sri Manakula Vinayagar Medical College and Hospital, Kalitheerthalkuppam, Madhagadipet Corresponding author: Dr. T. H. Dilip Kumar Date of submission: 12 May 2014 ; Date of Publication: 15 September 2014 Abstract: Introduction : Metopic suture is defined as a condition in which two pieces of frontal bone fail to merge in early childhood. Complete metopic suture extends from nasion to bregma and incomplete metopic suture present at the glabella of skull. It may be misdiagnosed as vertical traumatic skull fracture. Methods : This study was conducted in 50 skulls from the Department of Anatomy, Sri Manakula Vinayagar Medical College and Hospital, Pondicherry. Skulls with signs of disease and damaged skulls were excluded from the study. Metopic sutures are divided into three groups Absent, Complete and Incomplete sutures. Results : Complete metopic suture were present in 2% (1/50), Incomplete metopic suture were present in 44 %( 22/50) and Absent in 54 %( 27/50). Conclusion: The persistence of metopic suture in adults which separates the frontal bones are important in assessing the radiological images and in evaluation of medico legal cases. Keywords: Metopic suture, vertical fracture Introduction: Frontal bone is a curved plate of pneumatic bone 1. Metopic suture is formed between the tubers of frontal bone. Usually it is closed by the intramembranous ossification from the inner face of skull. The closure occurs from the two primary centres from each half of the frontal 2 bone . -

Morphology of Metopic Suture and Its Clinical Significance in Human Adult Skull
ORIGINAL ARTICLE Morphology of metopic suture and its clinical significance in human adult skull Sangeetha V1, Sundar G2 Sangeetha V, Sundar G. Morphology of metopic suture and its clinical Materials and Method: 70 dry adult skulls were observed for the presence of significance in human adult skull. Int J Anat Var. 2018;11(2): 40-42. metopic suture. Metopic suture were classified into complete metopic suture (metopism) and incomplete metopic suture type. SUMMARY Results: In the present study the incidence of metopism was 5.71% in South Introduction: Metopic suture is a dentate type of suture extending from Indian population. the nasion to the bregma of the skull bone.It is otherwise known as median frontal suture. The metopic suture normally closes at the age of 8 years Conclusion: The knowledge of metopic suture is significant for radiologist sometime even after 8 years it persists due to non- union of two halve of (which is usually mistaken as cranial fracture), neurosurgeons, forensic frontal bones.The incidence of metopism varies with race.Hence the present medicine and anthropologist. study was undertaken. Key Words: Suture; Metopism; Frontal bone; Nasion; Bregma Aim of the Study: To find out the incidence of metopism in South Indian population. INTRODUCTION Department of Anatomy, Subbaiah Institute of Medical Sciences and Govt VelloreMedical College. The non-mutilated complete adult skull examined rontal bone is a pneumatic single flat bone of the calvaria. It has a two for metopic suture.The metopic suture classification followed by Agarwal Fparts, squamous part involved in the formation of forehead whereas et al., (7) Ajmani et al., (11) and Castilho et al., (12) were applied. -

Dimensions and Ossification of the Normal Anterior Cranial Fossa In
Dimensions and Ossification of the Normal ORIGINAL RESEARCH Anterior Cranial Fossa in Children D.C. Hughes BACKGROUND AND PURPOSE: Interpretation of CT of the anterior skull base in children depends on M.J. Kaduthodil knowledge of the pattern and chronology of ossification. The purpose of this study was to ascertain the age at which the anterior cranial fossa is fully ossified as assessed on CT examinations. D.J.A. Connolly P.D. Griffiths MATERIALS AND METHODS: This was a retrospective review of 127 CT examinations of children ranging from 1 day to 16 years 7 months of age without known or suspected anterior cranial fossa abnormality. Measurements of the length and width of the anterior skull base and the presence and size of the most anterior unossified portion were determined by 2 investigators. RESULTS: At birth, the anterior skull base consists mainly of cartilage. There is a wide variation in ossification rates between individuals, but the anterior skull base was fully ossified at 3 years 10 months in all of our cases. CONCLUSIONS: In healthy individuals, the anterior skull base is fully ossified by 4 years of age. ABBREVIATIONS: cg ϭ crista galli; cp ϭ cribriform plate; f ϭ frontal bones; fc ϭ foramen cecum; hp ϭ hard palate: p ϭ perpendicular plate of the ethmoid bone; s ϭ sphenoid bone n important indication for imaging the anterior skull base computerized search. One hundred thirty-five patients were identi- Ais the evaluation of developmental midline masses fied initially, but after review of the clinical details from the imaging such as nasal dermal sinuses, anterior cephaloceles, and nasal requests and imaging reports, 8 patients with known metabolic or 1-3 gliomas. -

Title a Morphological Analysis of the Pterion in Genus Alouatta Author(S
Title A Morphological Analysis of the Pterion in Genus Alouatta Author(s) Mouri, Toshio Kyoto University overseas research reports of new world Citation monkeys (1984), 4: 29-34 Issue Date 1984 URL http://hdl.handle.net/2433/198755 Right Type Article Textversion publisher Kyoto University 29 Kyoto Kyoto University Overseas Research Reports Reports of New World Monkeys (1984) 4: 29 - 34 Kyoto Kyoto University Primate Research Institute A Morhpological Analysis of the Pterion in Genus Alouatta Toshia Toshia Mouri Primate Primate Research Institute, Kyoto University Pterion Pterion has gathered considerable attention in primate morphology, because of its high power characterizing New World Monkey (e.g. Rosenberger, 1977). In platyrrhine primates , pterion pterion is composed of frontal, sphenoidal, parietal and zygomatic bones, while in catarrhines , composed of frontal, sphenoidal, parietal and temporal bones. Furthermore, platyrrhine pterion pterion can be classified into two types according to its sutural pattern; spheno-frontal articu- lation lation type (SF type) pterion and parieto-zygomatic articulation type (PZ type) pterion. In the the extensive study on primate pterion by Ashley-Montagu (1933) , all callitrichids and 83.8 % of of cebids have PZ type pterions, while only the small portion (14.8 %) of cebids has SF type pterion. pterion. But SF type pterion occurred in about 60 % of Alouatta , highest incidence in Cebi- dae, dae, to say nothing of Ceboidea. The purpose of this study is to expain the high incidence of of SF type pterion in Alouatt α, analyzing the relation between incidences of SF type pterion and cranial mid-sagittal measurements of subsamples set up on the ground of age , sex and specific specific distinctions. -

Anatomy of the Pterygopalatine Fossa: an Innovative Metrical Assessment Based on 3D Segmentation on Head CT-Scan
Surgical and Radiologic Anatomy (2019) 41:523–528 https://doi.org/10.1007/s00276-018-2153-7 ORIGINAL ARTICLE Anatomy of the pterygopalatine fossa: an innovative metrical assessment based on 3D segmentation on head CT-scan Daniele Gibelli1 · Michaela Cellina2 · Stefano Gibelli3 · Annalisa Cappella1 · Marta Maria Panzeri4 · Antonio Giancarlo Oliva2 · Giovanni Termine3 · Claudia Dolci1 · Chiarella Sforza1 Received: 1 September 2018 / Accepted: 7 December 2018 / Published online: 12 December 2018 © Springer-Verlag France SAS, part of Springer Nature 2018 Abstract Purpose The pterygopalatine fossa is an important anatomical structure for several surgical and anaesthesiologic procedures; yet, very few data are available about its size. This study aims at providing a metrical assessment of pterygopalatine fossa through an innovative 3D segmentation procedure on head CT-scans. Methods CT-scans from 100 patients (50 males and 50 females) aged between 18 and 85 years were chosen for the study. Right and left pterygopalatine fossae were segmented through ITK-SNAP open source software. Height and volume were calculated on the acquired 3D models. In addition, anterior–posterior nasal spine distance, upper facial height (nasion–pros- thion) and biorbital breadth (ectoconchion–ectoconchion) were measured as well. Statistically significant differences of height and volume according to sex and side were assessed through two-way ANOVA test: sexually dimorphic measurements were further assessed through one-way ANCOVA test using the three cranial measurements as covariates (p < 0.05). Results On average pterygopalatine fossa height was 24.1 ± 3.5 mm in males, and 22.8 ± 3.4 mm in females, whereas volume was 0.930 ± 0.181 cm3 in males and 0.817 ± 0.157 cm3 in females, with statistically significant differences according to sex (p < 0.05), but not to side (p > 0.05); interaction was negligible for both the measurements.