Download Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Activity Budget and Spatial Behavior of the Emerald Tree Boa Corallus Batesii
Activity Budget and Spatial Behavior of the Emerald Tree Boa Corallus batesii Faculty Member #1 Joseph R. Mendelson III Signature Faculty Member #2 Emily G. Weigel Signature 2 Acknowledgments I would first like to thank my primary research advisor, Professor Joseph Mendelson, for your guidance and support. Thank you for inviting me to be a part of the emerald boa project and for investing so much time in helping me to become a scientist. I would also like to thank my second research advisor, Professor Emily Weigel, for helping me to get involved in research. Thank you for all of your help with my statistics and analysis and for providing detailed feedback to help me improve my scientific writing. Next, I would like to thank members of my research team: Liz Haseltine, Sav Berry, and Ellen Sproule. Thank you for organizing this study and for your help analyzing our 1,104 hours of video footage. I would like to thank members of the Spatial Ecology and Paleontology lab for your help in training me to become a better researcher. Thank you to Professor Jenny McGuire, Dr. Sílvia Pineda-Munoz, Dr. Yue Wang, Dr. Rachel Short, and Julia Schap. A special thanks to Ben Shipley and Danny Lauer for teaching me how to use R. Finally, I would like to thank my family for your continuous support while I study to become a wildlife biologist. Thank you for listening to me talk about snakes for the past few years. 3 Abstract Corallus batesii is a boid snake native to the Amazon basin. -

De Los Reptiles Del Yasuní
guía dinámica de los reptiles del yasuní omar torres coordinador editorial Lista de especies Número de especies: 113 Amphisbaenia Amphisbaenidae Amphisbaena bassleri, Culebras ciegas Squamata: Serpentes Boidae Boa constrictor, Boas matacaballo Corallus hortulanus, Boas de los jardines Epicrates cenchria, Boas arcoiris Eunectes murinus, Anacondas Colubridae: Dipsadinae Atractus major, Culebras tierreras cafés Atractus collaris, Culebras tierreras de collares Atractus elaps, Falsas corales tierreras Atractus occipitoalbus, Culebras tierreras grises Atractus snethlageae, Culebras tierreras Clelia clelia, Chontas Dipsas catesbyi, Culebras caracoleras de Catesby Dipsas indica, Culebras caracoleras neotropicales Drepanoides anomalus, Culebras hoz Erythrolamprus reginae, Culebras terrestres reales Erythrolamprus typhlus, Culebras terrestres ciegas Erythrolamprus guentheri, Falsas corales de nuca rosa Helicops angulatus, Culebras de agua anguladas Helicops pastazae, Culebras de agua de Pastaza Helicops leopardinus, Culebras de agua leopardo Helicops petersi, Culebras de agua de Peters Hydrops triangularis, Culebras de agua triángulo Hydrops martii, Culebras de agua amazónicas Imantodes lentiferus, Cordoncillos del Amazonas Imantodes cenchoa, Cordoncillos comunes Leptodeira annulata, Serpientes ojos de gato anilladas Oxyrhopus petolarius, Falsas corales amazónicas Oxyrhopus melanogenys, Falsas corales oscuras Oxyrhopus vanidicus, Falsas corales Philodryas argentea, Serpientes liana verdes de banda plateada Philodryas viridissima, Serpientes corredoras -

Zootaxa, Molecular Phylogeny, Classification, and Biogeography Of
Zootaxa 2067: 1–28 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) Molecular phylogeny, classification, and biogeography of West Indian racer snakes of the Tribe Alsophiini (Squamata, Dipsadidae, Xenodontinae) S. BLAIR HEDGES1, ARNAUD COULOUX2, & NICOLAS VIDAL3,4 1Department of Biology, 208 Mueller Lab, Pennsylvania State University, University Park, PA 16802-5301 USA. E-mail: [email protected] 2Genoscope. Centre National de Séquençage, 2 rue Gaston Crémieux, CP5706, 91057 Evry Cedex, France www.genoscope.fr 3UMR 7138, Département Systématique et Evolution, Muséum National d’Histoire Naturelle, CP 26, 57 rue Cuvier, 75005 Paris, France 4Corresponding author. E-mail : [email protected] Abstract Most West Indian snakes of the family Dipsadidae belong to the Subfamily Xenodontinae and Tribe Alsophiini. As recognized here, alsophiine snakes are exclusively West Indian and comprise 43 species distributed throughout the region. These snakes are slender and typically fast-moving (active foraging), diurnal species often called racers. For the last four decades, their classification into six genera was based on a study utilizing hemipenial and external morphology and which concluded that their biogeographic history involved multiple colonizations from the mainland. Although subsequent studies have mostly disagreed with that phylogeny and taxonomy, no major changes in the classification have been proposed until now. Here we present a DNA sequence analysis of five mitochondrial genes and one nuclear gene in 35 species and subspecies of alsophiines. Our results are more consistent with geography than previous classifications based on morphology, and support a reclassification of the species of alsophiines into seven named and three new genera: Alsophis Fitzinger (Lesser Antilles), Arrhyton Günther (Cuba), Borikenophis Hedges & Vidal gen. -

Boidae, Boinae): a Rare Snake from the Vale Do Ribeira, State of São Paulo, Brazil
SALAMANDRA 47(2) 112–115 20 May 2011 ISSNCorrespondence 0036–3375 Correspondence New record of Corallus cropanii (Boidae, Boinae): a rare snake from the Vale do Ribeira, State of São Paulo, Brazil Paulo R. Machado-Filho 1, Marcelo R. Duarte 1, Leandro F. do Carmo 2 & Francisco L. Franco 1 1) Laboratório de Herpetologia, Instituto Butantan, Av. Vital Brazil, 1500, São Paulo, SP, CEP: 05503-900, Brazil 2) Departamento de Agroindústria, Alimentos e Nutrição. Escola Superior de Agronomia “Luiz de Queiroz” – ESALQ/USP, Av. Pádua Dias, 11 C.P.: 9, Piracicaba, SP, CEP: 13418-900, Brazil Correspondig author: Francisco L. Franco, e-mail: [email protected] Manuscript received: 9 December 2010 The boid genusCorallus Daudin, 1803 is comprised of nine Until recently, only four specimens (including the above Neotropical species (Henderson et al. 2009): Corallus an mentioned holotype) of C. cropanii were deposited in her- nulatus (Cope, 1876), Corallus batesii (Gray, 1860), Co petological collections: three in the Coleção Herpetológica rallus blombergi (Rendahl & Vestergren, 1941), Coral “Alphonse Richard Hoge”, Instituto Butantan, São Paulo, lus caninus (Linnaeus, 1758), Corallus cookii Gray, 1842, Corallus cropanii (Hoge, 1954), Corallus grenadensis (Bar- bour, 1914), Corallus hortulanus (Linnaeus, 1758), and Corallus ruschenbergerii (Cope, 1876). The most conspic- uous morphological attributes of representatives of these species are the laterally compressed body, robust head, slim neck, and the presence of deep pits in some of the la- bial scales (Henderson 1993a, 1997). Species of Corallus are distributed from northern Central American to south- ern Brazil, including Trinidad and Tobago and islands of the south Caribbean. Four species occur in Brazil: Corallus batesii, C. -

Attacked from Above and Below, New Observations of Cooperative and Solitary Predators on Roosting Cave Bats
bioRxiv preprint doi: https://doi.org/10.1101/550582; this version posted February 17, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Attacked from above and below, new observations of cooperative and solitary predators on roosting cave bats Krizler Cejuela. Tanalgo1, 2, 3, 7*, Dave L. Waldien4, 5, 6, Norma Monfort6, Alice Catherine Hughes1, 3* 1Landscape Ecology Group, Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 2International College, University of Chinese Academy of Sciences, Beijing 100049, People’s Republic of China 3Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China 4Harrison Institute, Bowerwood House, 15 St Botolph’s Road, Sevenoaks, Kent, TN13 3AQ, United Kingdom 5Christopher Newport University, 1 Avenue of the Arts, Newport News, VA 23606, United States of America 6Philippine Bats for Peace Foundation Inc., 5 Ramona Townhomes, Guadalupe Village, Lanang, Davao City 7Department of Biological Sciences, College of Arts and Sciences, University of Southern Mindanao, Kabacan 9407, North Cotabato, the Republic of the Philippines Corresponding authors: ACH ([email protected]) and KCT ([email protected]) Landscape Ecology Group, Centre for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Mengla, Yunnan Province 666303, People’s Republic of China Abstract Predation of bats in their roosts has previously only been attributed to a limited number of species such as various raptors, owls, and snakes. -
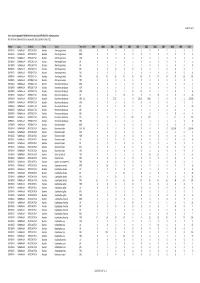
Gross Trade in Appendix II FAUNA (Direct Trade Only), 1999-2010 (For
AC25 Inf. 5 (1) Gross trade in Appendix II FAUNA (direct trade only), 1999‐2010 (for selection process) N.B. Data from 2009 and 2010 are incomplete. Data extracted 1 April 2011 Phylum Class TaxOrder Family Taxon Term Unit 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 Total CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BOD 0 00001000102 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia BON 0 00080000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia HOR 0 00000110406 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia LIV 0 00060000006 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKI 1 11311000008 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKP 0 00000010001 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia SKU 2 052101000011 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Ammotragus lervia TRO 15 42 49 43 46 46 27 27 14 37 26 372 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Antilope cervicapra TRO 0 00000020002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae BOD 0 00100001002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOP 0 00200000002 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae HOR 0 0010100120216 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae LIV 0 0 5 14 0 0 0 30 0 0 0 49 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA KIL 0 5 27.22 0 0 272.16 1000 00001304.38 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae MEA 0 00000000101 CHORDATA MAMMALIA ARTIODACTYLA Bovidae Bison bison athabascae -

Calabaria and the Phytogeny of Erycine Snakes
<nological Journal of the Linnean Socieb (1993), 107: 293-351. With 19 figures Calabaria and the phylogeny of erycine snakes ARNOLD G. KLUGE Museum of <oolog~ and Department of Biology, University of Michigan, Ann Arbor, Mr 48109 U.S.A. Receiued October 1991, revised manuscript accepted Mar I992 Two major subgroups of erycine snakes, designated Charina and Eyx, are delimited with a cladistic analysis of 75 morphological characters. The hypotheses of species relationships within the two clades are (reinhardtii (bottae, triuirgata) ) and (colubrinus, conicus, elegans, jayakari, muellen’, somalicus (miliaris (tataricus (iaculus, johnii)))),respectively. This pattern of grouping obtains without assuming multistate character additivity. At least 16 synapomorphies indicate that reinhardtii is an erycine and that it is the sister lineage of the (bottae, friuirgata) cladr. Calabaria and Lichanura are synonymized with Charina for reasons of taxonomic efficiency, and to emphasize the New-Old World geographic distribution of the three species in that assemblage. Further resolution of E’yx species relationships is required before Congylophis (type species conicus) can be recognized. ADDITIONAL KEY WORDS:--Biogeography - Cladistics - erycines - fossils - taxonomy CONI‘EN’I’S Introduction ................... 293 Erycine terminal taxa and nomenclature ............ 296 Fossils .................... 301 Methods and materials ................ 302 Eryrine phylogeny ................. 306 Character descriptions ............... 306 Other variation ................ -

Squamata: Tropidophiidae)
caribbean herpetology note Easternmost record of the Cuban Broad-banded Trope, Tropidophis feicki (Squamata: Tropidophiidae) Tomás M. Rodríguez-Cabrera1*, Javier Torres2, and Ernesto Morell Savall3 1Sociedad Cubana de Zoología, Cuba. 2 Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, Kansas 66045, USA. 3Área Protegida “Sabanas de Santa Clara,” Empresa Nacional para la Protección de la Flora y la Fauna, Villa Clara 50100, Cuba. *Corresponding author ([email protected]) Edited by: Robert W. Henderson. Date of publication: 14 May 2020. Citation: Rodríguez-Cabrera TM, Torres J, Morell Savall E (2020) Easternmost record of the Cuban Broad-banded Trope, Tropidophis feicki (Squa- mata: Tropidophiidae), of Cuba. Caribbean Herpetology, 71, 1-3. DOI: https://doi.org/10.31611/ch.71 Tropidophis feicki Schwartz, 1957 is restricted to densely forested limestone mesic areas in western Cuba (Schwartz & Henderson 1991; Henderson & Powell 2009). This species has been reported from about 20 localities distributed from near Guane, in Pinar del Río Province, to Ciénaga de Zapata, in Matanzas Province Rivalta et al., 2013; GBIF 2020; Fig. 1). On 30 June 2009 and on 22 December 2011 we found an adult male and an adult female Tropidophis feicki (ca. 400 mm SVL; Fig. 2), respectively, at the entrance of the “Cueva de la Virgen” hot cave (22.8201, -80.1384; 30 m a.s.l.; WGS 84; point 14 in Fig. 1). The cave is located within “Mogotes de Jumagua” Ecological Reserve, Sagua La Grande Municipality, Villa Clara Province. This locality represents the first record of this species for central Cuba, particularly for Villa Clara Province. -

Scientific Survey of Porto Rico and the Virgin Islands
: NEW YORK ACADEMY OF SCIENCES SCIENTIFIC SURVEY OF Porto Rico and the Virgin Islands VOLUME X NEW YORK Published by the Academy 1930 CONTENTS OF VOLUME X Page Title-page. Contents ^ Dates of Publication of Parts " List of Illustrations iv Amphibians and Land Reptiles of Porto Rico, with a List of Those Reported from the Virgin Islands. By Karl Patterson Schmidt 1 The Fishes of Porto Rico and the Virgin Islands—Branchiostomidae to Sciae- nidae. By J. T. Nichols 161 The Fishes of Porto Rico and the Virgin Islands—Pomacentridae to Ogcoce- phaUdae. By. J. T. Nichols 297 The Ascidians of Porto Rico and the Virgin Islands. By Willard G. Van Name 401 3 Index 5 ' Dates of Publication of Parts Part 1, November 22, 1928. ^ Part 2, September 10, 1929. ^"^ *7 jL mL. Part 3, March 15, 1930 Part 4, August 1, 1930 (iii) 'X -«^- AMPHIBIANS AND LAND REPTILES OF PORTO RICO With a List of Those Reported from the Virgin Islands By Karl Patterson Schmidt contents Page Introduction 3 Itinerary and collections made 4 Other material examined 4 Plan of work 5 Acknowledgments 6 Porto Rican herpetology since 1904 6 Lists of the amphibians and land reptiles of Porto Rico and the adjacent islands 7 Habitat associations and faunal subdivisions 9 Origin and relations of the Porto Rican herpetological fauna 12 Systematic account of the species 30 Class Amphibia 30 Order SaUentia 30 Family Bufonidae 30 Key to the genera of Porto Rican frogs and toads 30 Bufo Laurenti 31 Key to the Porto Rican species of true toads 31 Bufo lemur (Cope) 31 Bufo marinus (Linne) 34 Leptodactylus -

Coordinated Hunting by Cuban Boas
ABC 2017, 4(1):24-29 Animal Behavior and Cognition DOI: 10.12966/abc.02.02.2017 ©Attribution 3.0 Unported (CC BY 3.0) Coordinated Hunting by Cuban Boas Vladimir Dinets1,* 1University of Tennessee, Knoxville, TN *Corresponding author (Email: [email protected]) Citation – Dinets, V. (2017). Coordinated hunting by Cuban boas. Animal Behavior and Cognition, 4(1), 24–29. doi: 10.12966/abc.02.02.2017 Abstract - Coordinated hunting, in which individual predators relate in time and space to each other’s actions, is uncommon in animals, and is often difficult to distinguish from simply hunting in non-coordinated groups, which is much more common. The author tested if Cuban boas (Chilabothrus angulifer) hunting bats in cave passages take into account other boas’ positions when choosing hunting sites, and whether their choices increase hunting efficiency. Snakes arriving to the hunting area were significantly more likely to position themselves in the part of the passage where other snakes were already present, forming a “fence” across the passage and thus more effectively blocking the flight path of the prey, significantly increasing hunting efficiency. This is the first study to test for coordination between hunting reptiles, rather than assume such coordination based on perceived complexity of hunting behavior. Keywords – Boidae, Chilabothrus angulifer, Cooperative hunting, Cuba, Predation, Social behavior Coordinated hunting, in which individual predators relate in time and space to each other’s actions, is uncommon but widespread in animals (Bailey, -

2019. Gada Darbības Pārskats
RĪGAS NACIONĀLAIS ZOOLOĢISKAIS DĀRZS 2O19 RĪGAS NACIONĀLAIS ZOOLOĢISKAIS DĀRZS 2019. GADĀ Rīgas zoodārzs atvērts 1912. gada 14. oktobrī. Paš- zā vairojās 15 EEP sugas, t.sk. trīs savvaļā izmirušas glie- reizējā teritorija – 20 ha. mežu partulu sugas. 1992. gadā Rīgas zoodārzs iestājās Eiropas Zoodār- 2019. gada beigās 79 sugas iekļautas Pasaules Sarkana- zu un akvāriju asociācijā – EAZA (European Associa- jā grāmatā kā apdraudētas (kategorijas VU, EN, CR, EW). tion of Zoos and Aquaria). 2019. gadā Rīgas zoodārzā vairojās 18 no tām. 1993. gadā izveidota Rīgas zoodārza filiāle “Cīruļi” Sīkāk par aizsargājamajām sugām zoodārza kolekcijā – Aizputes novada Kalvenes pagastā (pašreizējā terito- 70. lpp. rija – 132 ha). Zoodārzā notiek dzīvnieku uzvedības pētījumi, 2019. gadā sākti ekotoksikoloģiskie pētījumi. Apmeklētāji Sīkāk par pētījumiem zoodārzā – 21. lpp. Apmeklētāju skaits 2019. gadā – 327 403. Sugu saglabāšana un pētījumi dabā Jaunas ekspozīcijas Rīgas zoodārza līdzšinējais svarīgākais ieguldī jums Āfrikas Savannas ekspozīcija. bioloģiskās daudzveidības saglabāšanā ir 1988.– Invazīvo sugu ekspozīcijas – invazīvo bezmugur- 1992. gadā veiktā Eiropas kokvardes populācijas at- kaulnieku un zivju ekspozīcija Akvārijā un šakāļu ekspo - jaunošana Kurzemē. zīcija. 2019. gadā Rīgas zoodārzs uzsāka atjaunotās kokvaržu Sīkāk par būvdarbiem un remontdarbiem 2019. gadā – populācijas monitoringu. Zoodārzs aicināja Latvijas iedzī- 5. lpp. votājus ziņot par kokvaržu atradnēm, un zoodārza speciā- listi veica kokvaržu uzskaiti un ekoloģijas pētījumus. Dzīvnieku kolekcija Sīkāk par kokvaržu projektu – rakstā 51. lpp. 2019. gada 31. decembrī dzīvnieku kolekcijā bija 398 sugu 2314 dzīvnieki. Dzīvnieku rehabilitācija 2019. gadā vairojās 129 sugu dzīvnieki, piedzima vai 2019. gadā zoodārza karantīnā tika uzņemti 48 ne- izšķīlās 540 mazuļi. laimē nokļuvuši savvaļas dzīvnieki, kā arī 86 konfiscēti Sīkāk par dzīvnieku kolekciju – 22. -

Výroční Zpráva
2017 VÝROČNÍ ZPRÁVA Zoologická a botanická zahrada města Plzně / VÝROČNÍ ZPRÁVA 2017 Zoologická a botanická zahrada města Plzně Zoological and Botanical Garden Pilsen/ Annual Report 2017 Provozovatel ZOOLOGICKÁ A BOTANICKÁ ZAHRADA MĚSTA PLZNĚ, příspěvková organizace ZOOLOGICKÁ A BOTANICKÁ ZAHRADA MĚSTA PLZNĚ POD VINICEMI 9, 301 00 PLZEŇ, CZECH REPUBLIC tel.: 00420/378 038 325, fax: 00420/378 038 302 e-mail: [email protected], www.zooplzen.cz Vedení zoo Management Ředitel Ing. Jiří Trávníček Director Ekonom Jiřina Zábranská Economist Provozní náměstek Ing. Radek Martinec Assistent director Vedoucí zoo. oddělení Bc. Tomáš Jirásek Head zoologist Zootechnik Svatopluk Jeřáb Zootechnicist Zoolog Ing. Lenka Václavová Curator of monkeys, carnivores Jan Konáš Curator of reptiles Miroslava Palacká Curator of ungulates Botanický náměstek, zoolog Ing. Tomáš Peš Head botanist, curator of birds, small mammals Botanik Mgr. Václava Pešková Botanist Propagace, PR Mgr. Martin Vobruba Education and PR Sekretariát Alena Voráčková Secretary Privátní veterinář MVDr. Jan Pokorný Veterinary Celkový počet zaměstnanců Total Employees (k 31. 12. 2017) 130 Zřizovatel Plzeň, statutární město, náměstí Republiky 1, Plzeň IČO: 075 370 tel.: 00420/378 031 111 Fotografie: Kateřina Misíková, Jiří Trávníček, Tomáš Peš, Miroslav Volf, Martin Vobruba, Jiřina Pešová, archiv Zoo a BZ, DinoPark, Oživená prehistorie a autoři článků Redakce výroční zprávy: Jiří Trávníček, Martin Vobruba, Tomáš Peš, Alena Voráčková, Kateřina Misíková, Pavel Toman, David Nováček a autoři příspěvků 1 výroční