Assessment of Heavy Metals Inactivation in Contaminated Soil by Coal Fly and Bottom Ashes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heavy Metal Pollution and Ecological Assessment Around the Jinsha Coal-Fired Power Plant (China)
International Journal of Environmental Research and Public Health Article Heavy Metal Pollution and Ecological Assessment around the Jinsha Coal-Fired Power Plant (China) Xianfei Huang ID , Jiwei Hu *, Fanxin Qin, Wenxuan Quan, Rensheng Cao, Mingyi Fan ID and Xianliang Wu Guizhou Provincial Key Laboratory for Environment, Guizhou Normal University, Guiyang 550001, China; [email protected] (X.H.); [email protected] (F.Q.); [email protected] (W.Q.); [email protected] (R.C.); [email protected] (M.F.); [email protected] (X.W.) * Correspondence: [email protected]; Tel.: +86-851-8670-0996; Fax: +86-851-8670-2710 Received: 4 November 2017; Accepted: 13 December 2017; Published: 18 December 2017 Abstract: Heavy metal pollution is a serious problem worldwide. In this study, 41 soil samples and 32 cabbage samples were collected from the area surrounding the Jinsha coal-fired power plant (JCFP Plant) in Guizhou Province, southwest China. Pb, Cd, Hg, As, Cu and Cr concentrations in soil samples and cabbage samples were analysed to study the pollution sources and risks of heavy metals around the power plant. The results indicate that the JCFP Plant contributes to the Pb, Cd, As, Hg, Cu, and Cr pollution in nearby soils, particularly Hg pollution. Cu and Cr in soils from both croplands and forestlands in the study area derive mainly from crustal materials or natural processes. Pb, Cd and As in soils from croplands arise partly through anthropogenic activities, but these elements in soils from forestlands originate mainly from crustal materials or natural processes. Hg pollution in soils from both croplands and forestlands is caused mainly by fly ash from the JCFP Plant. -

Toxic Substances in the Environment Toxse 2017
Department of Agricultural and Environmental Chemistry Al PAH Mn ? Cu Zn Cd Cr Pb Ni VIII International Scientific Conference TOXIC SUBSTANCES IN THE ENVIRONMENT TOXSE 2017 Krakow, Poland, 14-15 September 2017 Book of Abstracts Department of Agricultural and Environmental Chemistry University of Agriculture in Krakow VIII International Scientific Conference TOXIC SUBSTANCES IN THE ENVIRONMENT Krakow, Poland, 14-15 September 2017 Book of Abstracts Edited by: Tomáš Lošák, Monika Tabak, Dawid Tabak, Jacek Antonkiewicz Computer type setting: Monika Tabak Copyright © 2017 by Department of Agricultural and Environmental Chemistry, University of Agriculture in Krakow Edition: 150 copies Printed by: AlejeStudio, Druk Cyfrowy/Druk Wielkoformatowy/Studio Graficzne, Al. Juliusza Słowackiego 1, 31-159 Kraków The abstracts have not been peer-reviewed; only minor editorial changes were introduced. Authors are responsible for the content and linguistic correctness of the abstracts. ISBN 978-83-948965-0-8 CONFERENCE ORGANISER Department of Agricultural and Environmental Chemistry of the University of Agriculture in Krakow PATRONAGE Prof. Włodzimierz Sady, PhD, DSc, Eng. – Rector of the University of Agriculture in Krakow, Poland Paweł Ciećko, MSc – Inspector of Environmental Protection of Malopolska Province, Poland Commision "Soil and Human Health" – Polish Society of Soil Science PARTNERS Polish Society of Ecological Engineering Krakow Branch Department of Agroenvironmental Chemistry and Plant Nutrition, Czech University of Life Sciences Prague Department of Environmentalistics and Natural Resources, Mendel University in Brno Department of Agrochemistry, Soil Science, Microbiology and Plant Nutrition, Mendel University in Brno Institute of Highway Technology and Innovation (IATI) - Competence Center: Technologies Helping Out Natural Management of Organic and Mineral Waste (TWPZO) SPONSORS SCIENTIFIC COMMITTEE Prof. -

E-Brochure About Rybnik and Lower Secondary School No 18 in Rybnik
Zespół Szkół nr 2 Gimnazjum Dwujęzyczne nr 18 w Rybniku E-brochure about Rybnik and Lower Secondary School no 18 in Rybnik This project has been funded with support from the European Commission. This publication [communication] reflects the views only of the author, and the Commission cannot be held responsible for any use which may be made of the information contained therein. There is a school in this building since 15th March 1935. At first, it was a primary school, but during the Second World War the building served as hospital for soldiers. After war, there was a primary and a sec- ondary school only for girls in the building. Since 1963, both boys and girls can attend the secondary school. Our school has existed since 2008. Then, a unit of two schools – Secondary School no 2 and Lower Secondary School no 18 was created. There are about 95 students in the lower secondary school. We learn in bilingual classes, where apart from five lessons of English a week, we have history and IT lessons taught partly in English. Apart from English language, we learn German as well. In the whole Unit of Schools there are about 50 teachers, 22 classes and 700 students. It is possible to learn English, German, French, Italian or Russian. High school classes have specializations, such as maths and physics, biology and chemistry, history and maths, maths and geography. Moreover, students of second year can choose classes of dancing or swimming as their PE lessons. Virtual tour of our school This is our assembly hall. -
1A Lista Ref CBKK Ang 2005
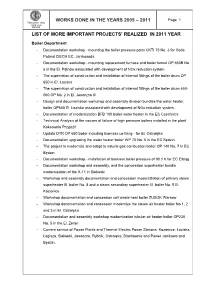
WORKS DONE IN THE YEARS 2005 – 2011 Page 1 Tarnowskie Góry Capital Group SEFAKO LIST OF MORE IMPORTANT PROJECTS’ REALIZED IN 2011 YEAR Boiler Department - Documentation workshop - mounting the boiler pressure parts CKTI 75 No. 3 for Soda Poland CIECH EC. Janikosoda - Documentation workshop - mounting replacement furnace and boiler funnel OP 650B No. 5 in the El. P ątnów associated with development of NOx reduction system. - The supervision of construction and installation of internal fittings of the boiler drum OP 650 in El. Łaziska - The supervision of construction and installation of internal fittings of the boiler drum 650- 060 OP No. 2 in El. Jaworzno III - Design and documentation workshop and assembly division bundles the water heater, boiler OP650 El. Łaziska associated with development of NOx reduction system. - Documentation of modernization BFB 100 boiler water heater in the EC Czechnica - Technical Analysis of the causes of failure of high pressure boilers installed in the plant Koksownia Przyja źń - Update DTR OP 650 boiler including biomass co-firing - for El. Ostroł ęka - Documentation upgrading the water heater boiler WP 70 No. 5 in the EC B ędzin - The project to modernize and adapt to natural gas combustion boiler OP 140 No. 7 in EC Będzin - Documentation workshop - installation of biomass boiler pressure of 90 t/ h for EC Elbl ąg - Documentation workshop and assembly, and the concession superheater bundle modernization of the K-11 in Siekierki - Workshop and assembly documentation and concession modernization of primary steam superheater III boiler No. 8 and a steam secondary superheater III boiler No. 5 El. -

Railways 02/2012
THE DB SCHENKER RAIL CUSTOMER MAGAZINE NO. 02 | 12 NORTH RAIL EXPRESS BRENNER GREAT BRITAIN Fisherman’s Up Island Solutions – Friend and under even for the Queen Page 16 Page 20 Page 28 Service & quality DB Schenker Rail is shifting focus – on being closer to its customers, on greater efficiency and greater punctuality Page 08 Heavier, higher, further As the athletes gear up for the Olympics in London, we have already finished our preparations for the summer games, having taken some 4,000 goods wagons to the venues in and around London carrying a total of around three million tonnes of construction materials. That’s why we alter the Olympic motto slightly, changing it to higher, further, heavier – then the sporting creed can apply to us here at DB Schenker Rail, too. We have gone higher, 1,370 metres above sea level to be exact, over the Brenner, the two reasons for which are illustrated by a large infographic in this issue of railways. And we want to take our customers further, by optimising our processes and increasing quality and service throughout the entire line. You can find out more in the Focus section from page 8 onwards. Michael Neuhaus; Kai Hartmann/Deutsche Bahn AG AG Hartmann/Deutsche Bahn Michael Neuhaus; Kai I wish you an exciting read! A passion for steel / Photos: very coil is different, and each one platforms in the shed. In general, four Best regards, has not only a number, but also a freight trains are loaded with the heavy E story,” says Anton Blaj. The 54- semi-finished product each day, which year-old loadmaster at the state-of-the-art ThyssenKrupp Steel Europe supplies to continuous casting and rolling plant oper- the automotive, electronics and construc- ated by ThyssenKrupp Steel Europe in tion industries for further processing. -

Prezentacja Programu Powerpoint
About Us Dear Sirs, DSBJ Advisory Group (DSBJ Grupa Doradcza Sp. z o.o., further DSBJ) operates in many areas of consulting and offers full range of services in the area of transactions on capital markets and provides advisory services in economic and strategic consulting, equity and debt financing, building a strategic development strategy, corporate image creation, advising on corporate finance and the financing sourced from European grants. DSBJ operates since April 2009 and in present form since March 2011. The origin of activity dates back to 1993. Its experience gained since the early nineties focuses, among others, on the following areas: development of retail chains food industry high-tech industry environmental protection ICT On 7 July, 2011, pursuant to the resolutions of the Board of the Warsaw Stock Exchange DSBJ acquired the status of an Authorised Adviser on the NewConnect stock market and Catalyst bond market. Thanks to this DSBJ have been able to expand service range with comprehensive services for companies on securities markets. Bond issue is an excellent way to obtain financing, and it has opened the Polish capital market not only to small and medium-sized enterprises (Polish and foreign) but also local government units (municipal bonds). DSBJ Grupa Doradcza Sp. z o.o., office: ul. Jan Nowak-Jeziorański 7/133, 03-984 Warsaw, Board of Directors: President Jerzy Z. Bednarek, Vice President Agnieszka Grabus, tel./fax: 0048 22 1151078, District Court for the Capital City of Warsaw, XII Commercial Division of the National Court Register KRS number: 0000389940, share capital: 6.500,00 PLN, BRE Bank SA 79 www.dsbj.eu 1140 2017 0000 4902 1266 7376 About Us It is worth noting that DSBJ’s activity often refers to business services as a “boutique advisory" which illustrates approach to collaboration with clients based on the principles of flexibility, integrity and exclusivity. -

Gas at a Crossroads: Why the EU Should Not Continue To
Gas at a Crossroads WHY THE EU SHOULD NOT CONTINUE TO EXPAND ITS GAS INFRASTRUCTURE Mason Inman GAS AT A CROSSROADS ABOUT THE COVER PERMISSIONS/COPYRIGHT The Adriatic Rovigo LNG Terminal. Courtesy of This publication may be reproduced in whole or in part and Floydrosebridge via Wikipedia (CC BY-SA 3.0). in any form for educational or nonprofit purposes without special permission from the copyright holders, provided ABOUT GLOBAL that acknowledgement of the source is made. No use of this Global publication may be made for resale or other commercial Energy ENERGY MONITOR purpose without written permission of the copyright hold- Monitor Global Energy Monitor (GEM) ers. Copyright © February 2020 by Global Energy Monitor. is a network of researchers developing collaborative informational resources on fossil fuels and alternatives. Current projects include the Global FURTHER RESOURCES Coal Plant Tracker, the Global Fossil Infrastructure Tracker, To obtain primary data from the Europe Gas Tracker or the the CoalWire newsletter, the Global Coal Mine Tracker, the Global Fossil Infrastructure Tracker, contact Ted Nace Global Gas Plant Tracker, and GEM.wiki. ([email protected]). ABOUT THE EUROPE GAS TRACKER CORRECTION The Europe Gas Tracker is an online database that identifies, The original version of this report contained errors in the maps, describes, and categorizes gas extraction areas, gas- compilation of data for power plants, which led to the fired power plants, fossil gas pipelines, and liquified natural power plant capacity shown in Table 1 being doubled, and gas (LNG) terminals. Developed by Global Energy Monitor, the costs being increased by ~50% for many countries. -
Literature Review of Emissions and Environmental Effects of Coal
Literature review on atmospheric emissions and associated environmental effects from conventional thermal electricity generation Prepared for: Clean Air Strategic Alliance Electricity Project Team Prepared by: Heidi Swanson March 12, 2008 Table of Contents 1 Introduction................................................................................................................. 4 1.1 Objective and summary of literature review....................................................... 4 1.2 Literature search methodology ........................................................................... 4 1.2.1 Peer-Reviewed Journal Articles.................................................................. 4 1.2.2 Grey Literature Reports .............................................................................. 5 1.3 Organization of the literature review document ................................................. 6 1.4 Summary of findings and key studies................................................................. 7 1.4.1 Mercury....................................................................................................... 7 1.4.2 Sulphur dioxide........................................................................................... 8 1.4.3 Nitrogen oxides........................................................................................... 9 1.4.4 Particulate matter ........................................................................................ 9 1.4.5 List 2 substances (PAHs, particulate matter, trace metals, benzene, -

Heavy Metals in Fly Ash from a Coal-Fired Power Station in Poland
Polish J. of Environ. Stud. Vol. 15, No. 6 (2006), 943-946 Letter to Editor Heavy Metals in Fly Ash from a Coal-Fired Power Station in Poland D. Smołka-Danielowska university of Silesia, Faculty of earth Sciences, ul.Będzińska 60, 41-200 Sosnowiec, Poland Received: January 30, 2006 Accepted: July 28, 2006 Abstract The main subject of this study is fly ash created during coal combustion in Rybnik Power Station in Upper Silesia in the southern part of Poland. This is the biggest block power station in this region. Gener- ated power is 1600 MW. Annual dust emission from professional energy in Upper Silesia is estimated at 32.8 thousand tons, which is about 22% of total national emissions. X-ray powder diffraction, Analytic Scanning Electron Microscopy (ASEM) and Induced Couple Mass Spectrometry (ICP- MS) methods have been applied extensively to heavy metals (Cu, Ni, Pb, Zn, Cr, Cd) content analysis and their host mineral phases identification. Concentrations of Cu, Ni, Pb, Zn, Cr and Cd as well as mineral composition of fly ash being their carriers have been specified.�������������������������������������������������������������������� Average Zn concentrations in fly ash are 120 ppm, but for all other elements the averege concentrations were 38 ppm for Cu, 41 ppm for Ni, 44 ppm for Pb, 64 ppm for Cr and 3 ppm for Cd. Amorphic aluminosilicate substances, oxides and sulphides are mineral phases containing heavy metals. The sizes of these particles are up to 12 µm. Keywords: power station, fly ash, heavy metals Introduction fine dispersive dust and their various chemical composi- tions determines the toxic properties of air. -

Enbw Annual Report 2005
Annual Report 2005 Energy generates success At a glance EnBW group1 2005 2004 Variance % External sales Electricity € millions 8,150.2 7,088.9 +15.0 Gas € millions 2,101.7 1,540.6 +36.4 Energy and environmental services € millions 517.4 494.8 +4.6 External sales, total € millions 10,769.3 9,124.3 +18.0 EBITDA € millions 2,054.3 2,044.5 +0.5 EBIT € millions 1,318.5 1,242.5 +6.1 Result of continuing operations € millions 522.5 357.7 +46.1 Earnings per share from continuing operations € 2.17 1.57 +38.2 Cash flow from operating activities € millions 1,329.9 1,545.7 -14.0 Free cash flow € millions 1,070.6 1,330.8 -19.6 Capital expenditures on intangible assets and property, plant and equipment € millions 547.0 417.7 +31.0 Return on Capital Employed (ROCE) % 13.4 11.7 +14.5 Cost of capital rate (WACC) before tax % 9.0 9.0 – Average capital employed € millions 9,353.7 9,400.8 -0.5 Value added € millions 407.5 251.9 +61.8 Energy sales of the EnBW group1 2005 2004 Variance % Electricity billions of kWh 106.7 100.9 +5.7 Gas billions of kWh 88.6 82.9 +6.9 Employees of the EnBW group1,2 2005 2004 Variance % Employees (annual average) 17,926 19,881 -9.8 1 The prior-year figures have been adjusted (see comments on the change in reporting in the notes). 2 Number of employees without apprentices/trainees and without inactive employees. -
Katarzyna Wierzchowiecka Market Opportunities and Value Creation For
KATARZYNA WIERZCHOWIECKA MARKET OPPORTUNITIES AND VALUE CREATION FOR ENGINEERING SERVICES IN POLAND: THE MINING AND ENERGY INDUSTRIES Master of Science Thesis Prof. Olavi Uusitalo has been appointed as the examiner at the Council Meeting of the Faculty of Business and Built Environment on December the 3rd 2014. i ABSTRACT TAMPERE UNIVERSITY OF TECHNOLOGY Master’s Degree Programme in Industrial Engineering and Management WIERZCHOWIECKA, KATARZYNA: Market Opportunity and Value Creation for Engineering Services in Poland: The Mining and Energy Industries Master of Science Thesis, 89 pages, 9 appendices (10 pages) April 2015 Major: Industrial management Examiner: Professor Olavi Uusitalo Keywords: Market opportunity, Value creation, Poland The steadily accelerating globalization has given the means and motivation for companies to find new market opportunities and thus enter new markets. In order to minimize the risks involved in such activity and assess the size of the opportunity it is essential to conduct market opportunity analysis on the desired target market. Success in a market requires the knowledge on internal and external factors affecting market opportunity as well as the ability to understand target market’s needs and preferences. This way the customer value can be maximized and a mutually beneficial relationship is created. The objective of this thesis is to determine what kind of market opportunities there are for engineering services in the Polish energy and mining industries and how they should be pursued. The study was conducted as an empirical qualitative case study using referee-articles, Internet, qualitative interviews and surveys as a source of information. The framework created for this thesis can be used by companies to understand market opportunities in a given market and, thus, improve their value creation abilities and possibilities to succeed there. -

Optimization of Combustion Process in Coal-Fired Power Plant with Utilization of Acoustic System for In-Furnace Temperature Meas
Applied Thermal Engineering 123 (2017) 711–720 Contents lists available at ScienceDirect Applied Thermal Engineering journal homepage: www.elsevier.com/locate/apthermeng Research Paper Optimization of combustion process in coal-fired power plant with utilization of acoustic system for in-furnace temperature measurement ⇑ Łukasz S´ladewski a, , Konrad Wojdan a, Konrad S´wirski a, Tomasz Janda b, Daniel Nabagło b, Jerzy Chachuła b a Warsaw University of Technology, Institute of Heat Engineering, Nowowiejska 21/25 St., 00-665 Warszawa, Poland b EDF Polska CUW Sp. z o.o., Research & Development Department, Ciepłownicza 1 St., 31-587 Kraków, Poland highlights Novel approach in combustion modelling and optimization in coal-fired boiler. R&D and implementation project that was carried on on real boiler. Artificial immune optimization system integrated with temperature profile measurement. Proper control of the fireball shape resulted in 0.27% boiler efficiency increase. article info abstract Article history: This paper presents methodology and results of a research project on software optimization of combus- Received 26 November 2016 tion process efficiency in coal-fired power plant. Accepted 14 May 2017 The general goal of this project was to increase boiler efficiency by proper control of the combustion Available online 19 May 2017 process using optimization software, integrated with Distributed Control System and in-furnace temper- ature profile measurement system. The research goal relays on new approach in combustion modelling Keywords: based on in-furnace temperature distribution and utilization of this model in on-line boiler control. it is Combustion optimization assumed that this approach allows for more precise control of the combustion process, what finally has a Combustion advanced control positive influence on boiler performance – the efficiency in specific.