(DPP-4) Inhibitors Reference Number: CP.PMN.03 Effective Date: 09.19.18 Last Review Date: 02.21 Line of Business: Medicaid Revision Log
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metformin Plus Saxagliptin for Type 2 Diabetes
Treatment evaluation Metformin plus saxagliptin for type 2 diabetes André J. Scheen Division of Diabetes, Nutrition and Metabolic Disorders and Division of Clinical Pharmacology, Department of Medicine, CHU Sart Tilman, University of Liège, Liège, Belgium Running title : Saxagliptin plus metformin Word count : Abstract : 148 Main text : 2404 Tables : 4 Figures : 0 Address for correspondence : Pr André J. SCHEEN Department of Medicine CHU Sart Tilman (B35) B-4000 LIEGE 1 BELGIUM Phone : 32-4-3667238 FAX : 32-4-3667068 Page 1 Email : andre.scheen @ chu.ulg.ac.be SUMMARY Metformin is considered as the first-line drug therapy for the management of type 2 diabetes. Dipeptidyl peptidase-4 (DPP-4) inhibitors, by promoting insulin secretion and reducing glucagon secretion in a glucose-dependent manner, offer new opportunities for oral therapy after failure of metformin. Saxagliptin, a DPP-4 inhibitor, and metformin may be administered together, separately or in fixed-dose combination (FDC), either as saxagliptin added to metformin or as initial combination in drug-naive patients. Both compounds exert complementary pharmacodynamic actions leading to better improvement in blood glucose control (fasting plasma glucose, postprandial glucose, HbA1c) than either compound separately. Adding saxagliptin to metformin monthotherapy results in a consistent, sustained and safe reduction in HbA1c levels. Tolerance is excellent without hypoglycemia or weight gain. The combination saxaglitpin plus metformin may be used as first-line or second-line therapy in the management of type 2 diabetes, especially as a valuable alternative to the classical metformin-sulfonylurea combination. Key-words : DPP-4 inhibitor – Fixed-dose combination - Metformin – Saxagliptin - Type 2 diabetes mellitus Page 2 1. -

Rosiglitazone-Associated Fractures in Type 2 Diabetes an Analysis from a Diabetes Outcome Progression Trial (ADOPT)
Clinical Care/Education/Nutrition/Psychosocial Research ORIGINAL ARTICLE Rosiglitazone-Associated Fractures in Type 2 Diabetes An analysis from A Diabetes Outcome Progression Trial (ADOPT) 1 7 STEVEN E. KAHN, MB, CHB DAHONG YU, PHD preclinical data and better understand the 2 7 BERNARD ZINMAN, MD MARK A. HEISE, PHD clinical implications of and possible interven- 3 7 JOHN M. LACHIN, SCD R. PAUL AFTRING, MD, PHD tions for these findings. 4 8 STEVEN M. HAFFNER, MD GIANCARLO VIBERTI, MD 5 WILLIAM H. HERMAN, MD FOR THE ADIABETES OUTCOME Diabetes Care 31:845–851, 2008 6 RURY R. HOLMAN, MD PROGRESSION TRIAL (ADOPT) STUDY 7 BARBARA G. KRAVITZ, MS GROUP* ype 2 diabetes is associated with an increased risk of fractures, with the risk increasing with longer duration OBJECTIVE — The purpose of this study was to examine possible factors associated with the T increased risk of fractures observed with rosiglitazone in A Diabetes Outcome Progression Trial of disease (1,2). These fractures affect pre- (ADOPT). dominantly the hip, arm, and foot (1–5) and occur despite the fact that bone min- RESEARCH DESIGN AND METHODS — Data from the 1,840 women and 2,511 men eral density is either normal or even in- randomly assigned in ADOPT to rosiglitazone, metformin, or glyburide for a median of 4.0 years creased in patients with type 2 diabetes were examined with respect to time to first fracture, rates of occurrence, and sites of fractures. compared with those who are not hyper- glycemic (5–7). Although the reason for RESULTS — In men, fracture rates did not differ between treatment groups. -

CD26/DPP-4: Type 2 Diabetes Drug Target with Potential Influence On
cancers Review CD26/DPP-4: Type 2 Diabetes Drug Target with Potential Influence on Cancer Biology Emi Kawakita 1 , Daisuke Koya 2,3 and Keizo Kanasaki 1,3,* 1 Internal Medicine 1, Shimane University Faculty of Medicine, 89-1 Enya-cho, Izumo 693-8501, Japan; [email protected] 2 Department of Diabetology & Endocrinology, Kanazawa Medical University, Uchinada 920-0293, Japan; [email protected] 3 Division of Anticipatory Molecular Food Science and Technology, Medical Research Institute, Kanazawa Medical University, Uchinada 920-0293, Japan * Correspondence: [email protected]; Tel.: +81-853-20-2183 Simple Summary: Dipeptidyl peptidase (DPP)-4 inhibitor is widely used for type 2 diabetes. Al- though DPP-4/CD26 has been recognized as both a suppressor and inducer in tumor biology due to its various functions, how DPP-4 inhibitor affects cancer progression in diabetic patients is still unknown. The aim of this review is to summarize one unfavorable aspect of DPP-4 inhibitor in cancer-bearing diabetic patients. Abstract: DPP-4/CD26, a membrane-bound glycoprotein, is ubiquitously expressed and has diverse biological functions. Because of its enzymatic action, such as the degradation of incretin hormones, DPP-4/CD26 is recognized as the significant therapeutic target for type 2 diabetes (T2DM); DPP- 4 inhibitors have been used as an anti-diabetic agent for a decade. The safety profile of DPP-4 inhibitors for a cardiovascular event in T2DM patients has been widely analyzed; however, a clear association between DPP-4 inhibitors and tumor biology is not yet established. Previous preclinical Citation: Kawakita, E.; Koya, D.; Kanasaki, K. -

Diabetes Mellitus: Patterns of Pharmaceutical Use in Manitoba
Diabetes Mellitus: Patterns of Pharmaceutical Use in Manitoba by Kim¡ T. G. Guilbert A Thesis submitted to The Faculty of Graduate Studies in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Faculty of Pharmacy The University of Manitoba Winnipeg, Manitoba @ Kimi T.G. Guilbert, March 2005 TIIE UMYERSITY OF MANITOBA F'ACULTY OF GRADUATE STTJDIES +g+ù+ COPYRIGIIT PERMISSION PAGE Diabetes Mellitus: Patterns of Pharmaceutical Use in Manitoba BY Kimi T.G. Guilbert A ThesisÆracticum submitted to the Faculty of Graduate Studies of The University of Manitoba in partial fulfillment of the requirements of the degree of MASTER OF SCIENCE KIMI T.G. GTIILBERT O2()O5 Permission has been granted to the Library of The University of Manitoba to lend or sell copies of this thesis/practicum, to the National Library of Canada to microfïlm this thesis and to lend or sell copies of the film, and to University Microfilm Inc. to publish an abstract of this thesis/practicum. The author reserves other publication rights, and neither this thesis/practicum nor extensive extracts from it may be printed or otherwise reproduced without the author's written permission. Acknowledgements Upon initiation of this project I had a clear objective in mind--to learn more. As with many endeavors in life that are worthwhile, the path I have followed has brought me many places I did not anticipate at the beginning of my journey. ln reaching the end, it is without a doubt that I did learn more, and the knowledge I have been able to take with me includes a wider spectrum than the topic of population health and medication utilization. -

Identification of a Novel Selective Agonist of Pparc with No Promotion
OPEN Identification of a novel selective agonist c SUBJECT AREAS: of PPAR with no promotion of DRUG DISCOVERY ENDOCRINE SYSTEM AND adipogenesis and less inhibition of METABOLIC DISEASES osteoblastogenesis Received 28 October 2014 Chang Liu, Tingting Feng, Ningyu Zhu, Peng Liu, Xiaowan Han, Minghua Chen, Xiao Wang, Ni Li, Yongzhen Li, Yanni Xu & Shuyi Si Accepted 5 March 2015 Published Institute of Medicinal Biotechnology, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100050, China. 1 April 2015 Nuclear receptor peroxisome proliferator-activated receptor c (PPARc) plays an important role in the regulation of glucose homeostasis and lipid metabolism. However, current PPARc-targeting drugs such as Correspondence and thiazolidinediones (TZDs) are associated with undesirable side effects. We identified a small molecular requests for materials compound, F12016, as a selective PPARc agonist by virtual screening, which showed moderate PPARc should be addressed to agonistic activity and binding ability for PPARc. F12016 did not activate other PPAR subtypes at 30 mM and S.S. (sisyimb@hotmail. selectively modulated PPARc target gene expression. In diabetic KKAy mice, F12016 had insulin-sensitizing com) or Y.X. and glucose-lowering properties, and suppressed weight gain. In vitro, F12016 effectively increased glucose c (xuyanniwendeng@ uptake and blocked cyclin-dependent kinase 5-mediated phosphorylation of PPAR at Ser273, but slightly triggered adipogenesis and less inhibited osteoblastogenesis than rosiglitazone. Moreover, compared with hotmail.com) the full agonist rosiglitazone, F12016 had a distinct group of coregulators and a different predicted binding mode for the PPARc ligand-binding domain. A site mutation assay confirmed the key epitopes, especially Tyr473 in AF-2. -

Komboglyze, INN-Saxagliptin, Metformin
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Komboglyze 2.5 mg/850 mg film-coated tablets Komboglyze 2.5 mg/1,000 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Komboglyze 2.5 mg/850 mg film-coated tablets Each tablet contains 2.5 mg of saxagliptin (as hydrochloride) and 850 mg of metformin hydrochloride. Komboglyze 2.5 mg/1,000 mg film-coated tablets Each tablet contains 2.5 mg of saxagliptin (as hydrochloride) and 1,000 mg of metformin hydrochloride. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablet (tablet). Komboglyze 2.5 mg/850 mg film-coated tablets Light brown to brown, biconvex, round, film-coated tablets, with “2.5/850” printed on one side and “4246” printed on the other side, in blue ink. Komboglyze 2.5 mg/1,000 mg film-coated tablets Pale yellow to light yellow, biconvex, oval shaped, film-coated tablets, with “2.5/1000” printed on one side and “4247” printed on the other side, in blue ink. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Komboglyze is indicated in adults with type 2 diabetes mellitus as an adjunct to diet and exercise to improve glycaemic control: in patients inadequately controlled on their maximally tolerated dose of metformin alone in combination with other medicinal products for the treatment of diabetes, including insulin, in patients inadequately controlled with metformin and these medicinal products (see sections 4.4, 4.5 and 5.1 for available data on different combinations) in patients already being treated with the combination of saxagliptin and metformin as separate tablets. -
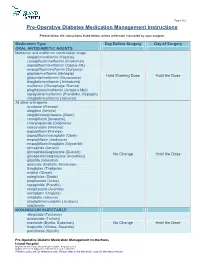
Pre-Operative Diabetes Medication Management Instructions
Page 1 of 2 Pre-Operative Diabetes Medication Management Instructions Please follow the instructions listed below unless otherwise instructed by your surgeon Medication Type Day Before Surgery Day of Surgery ORAL ANTIDIABETIC AGENTS Metformin and metformin combination drugs alogliptin/metformin (Kazano) canagliflozin/metformin (Invokamet) dapagliflozin/metformin (Xigduo XR) empagliflozin/metformin (Synjardy) glipizide/metformin (Metaglip) Hold Evening Dose Hold the Dose glyburide/metformin (Glucovance) linagliptin/metformin (Jentadueto) metformin (Glucophage, Riomet) pioglitazone/metformin (Actoplus Met) repaglidine/metformin (PrandiMet, Repaglin) sitagliptin/metformin (Janumet) All other oral agents acarbose (Precose) alogliptin (Nesina) alogliptin/pioglitazone (Oseni) canagliflozin (Invokana) chlorpropamide (Diabinese) colesevelam (Welchol) dapagliflozin (Farxiga) dapagliflozin/saxagliptin (Qtern) empagliflozin (Jardiance) empagliflozin/linagliptin (Glyxambi) glimepiride (Amaryl) glimepiride/pioglitazone (Duetact) No Change Hold the Dose glimepiride/rosiglitazone (Avandaryl) glipizide (Glucotrol) glyburide (DiaBeta, Micronase) linagliptan (Tradjenta) miglitol (Glyset) nateglinide (Starlix) pioglitazone (Actos) repaglinide (Prandin) rosiglitazone (Avandia) saxagliptin (Onglyza) sitagliptin (Januvia) sitagliptin/simvastatin (Juvisync) tolazamide NON-INSULIN INJECTABLE albiglutide (Tanzeum) dulaglutide (Trulicity) exenatide (Byetta, Bydureon) No Change Hold the Dose liraglutide (Victoza, Saxenda) pramlintide (Symlin) Pre-Operative Diabetic -

AVANDIA (Rosiglitazone Maleate Tablets), for Oral Use Ischemic Cardiovascular (CV) Events Relative to Placebo, Not Confirmed in Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION ----------------------- WARNINGS AND PRECAUTIONS ----------------------- These highlights do not include all the information needed to use • Fluid retention, which may exacerbate or lead to heart failure, may occur. AVANDIA safely and effectively. See full prescribing information for Combination use with insulin and use in congestive heart failure NYHA AVANDIA. Class I and II may increase risk of other cardiovascular effects. (5.1) • Meta-analysis of 52 mostly short-term trials suggested a potential risk of AVANDIA (rosiglitazone maleate tablets), for oral use ischemic cardiovascular (CV) events relative to placebo, not confirmed in Initial U.S. Approval: 1999 a long-term CV outcome trial versus metformin or sulfonylurea. (5.2) • Dose-related edema (5.3) and weight gain (5.4) may occur. WARNING: CONGESTIVE HEART FAILURE • Measure liver enzymes prior to initiation and periodically thereafter. Do See full prescribing information for complete boxed warning. not initiate therapy in patients with increased baseline liver enzyme levels ● Thiazolidinediones, including rosiglitazone, cause or exacerbate (ALT >2.5X upper limit of normal). Discontinue therapy if ALT levels congestive heart failure in some patients (5.1). After initiation of remain >3X the upper limit of normal or if jaundice is observed. (5.5) AVANDIA, and after dose increases, observe patients carefully for signs • Macular edema has been reported. (5.6) and symptoms of heart failure (including excessive, rapid weight gain; • Increased incidence of bone fracture was observed in long-term trials. dyspnea; and/or edema). If these signs and symptoms develop, the heart (5.7) failure should be managed according to current standards of care. -

OSENI (Alogliptin and Pioglitazone) Tablets II May Increase Risk
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------WARNINGS AND PRECAUTIONS--------------------- These highlights do not include all the information needed to use • Congestive heart failure: Fluid retention may occur and can OSENI safely and effectively. See full prescribing information for exacerbate or lead to congestive heart failure. Combination use OSENI. with insulin and use in congestive heart failure NYHA Class I and OSENI (alogliptin and pioglitazone) tablets II may increase risk. Monitor patients for signs and symptoms. Initial U.S. Approval: 2013 (5.1) • Acute pancreatitis: There have been postmarketing reports of WARNING: CONGESTIVE HEART FAILURE acute pancreatitis. If pancreatitis is suspected, promptly See full prescribing information for complete boxed warning discontinue OSENI. (5.2) • Thiazolidinediones, including pioglitazone, cause or • Hypersensitivity: There have been postmarketing reports of exacerbate congestive heart failure in some patients. (5.1) serious hypersensitivity reactions in patients treated with alogliptin • After initiation of OSENI and after dose increases, monitor such as anaphylaxis, angioedema and severe cutaneous adverse patients carefully for signs and symptoms of heart failure reactions. In such cases, promptly discontinue OSENI, assess for (e.g., excessive, rapid weight gain, dyspnea and/or other potential causes, institute appropriate monitoring and edema). If heart failure develops, it should be managed treatment and initiate alternative treatment for diabetes. (5.3) according to current standards of care and • Hepatic effects: Postmarketing reports of hepatic failure, discontinuation or dose reduction of pioglitazone in OSENI sometimes fatal. Causality cannot be excluded. If liver injury is must be considered. detected, promptly interrupt OSENI and assess patient for • OSENI is not recommended in patients with symptomatic probable cause, then treat cause if possible, to resolution or heart failure. -

Type 2 Diabetes Adult Outpatient Insulin Guidelines
Diabetes Coalition of California TYPE 2 DIABETES ADULT OUTPATIENT INSULIN GUIDELINES GENERAL RECOMMENDATIONS Start insulin if A1C and glucose levels are above goal despite optimal use of other diabetes 6,7,8 medications. (Consider insulin as initial therapy if A1C very high, such as > 10.0%) 6,7,8 Start with BASAL INSULIN for most patients 1,6 Consider the following goals ADA A1C Goals: A1C < 7.0 for most patients A1C > 7.0 (consider 7.0-7.9) for higher risk patients 1. History of severe hypoglycemia 2. Multiple co-morbid conditions 3. Long standing diabetes 4. Limited life expectancy 5. Advanced complications or 6. Difficult to control despite use of insulin ADA Glucose Goals*: Fasting and premeal glucose < 130 Peak post-meal glucose (1-2 hours after meal) < 180 Difference between premeal and post-meal glucose < 50 *for higher risk patients individualize glucose goals in order to avoid hypoglycemia BASAL INSULIN Intermediate-acting: NPH Note: NPH insulin has elevated risk of hypoglycemia so use with extra caution6,8,15,17,25,32 Long-acting: Glargine (Lantus®) Detemir (Levemir®) 6,7,8 Basal insulin is best starting insulin choice for most patients (if fasting glucose above goal). 6,7 8 Start one of the intermediate-acting or long-acting insulins listed above. Start insulin at night. When starting basal insulin: Continue secretagogues. Continue metformin. 7,8,20,29 Note: if NPH causes nocturnal hypoglycemia, consider switching NPH to long-acting insulin. 17,25,32 STARTING DOSE: Start dose: 10 units6,7,8,11,12,13,14,16,19,20,21,22,25 Consider using a lower starting dose (such as 0.1 units/kg/day32) especially if 17,19 patient is thin or has a fasting glucose only minimally above goal. -

AVANDIA® (Rosiglitazone Maleate) Tablets
PRESCRIBING INFORMATION AVANDIA® (rosiglitazone maleate) Tablets WARNING: CONGESTIVE HEART FAILURE ● Thiazolidinediones, including rosiglitazone, cause or exacerbate congestive heart failure in some patients (see WARNINGS). After initiation of AVANDIA, and after dose increases, observe patients carefully for signs and symptoms of heart failure (including excessive, rapid weight gain, dyspnea, and/or edema). If these signs and symptoms develop, the heart failure should be managed according to current standards of care. Furthermore, discontinuation or dose reduction of AVANDIA must be considered. ● AVANDIA is not recommended in patients with symptomatic heart failure. Initiation of AVANDIA in patients with established NYHA Class III or IV heart failure is contraindicated. (See CONTRAINDICATIONS and WARNINGS.) DESCRIPTION AVANDIA (rosiglitazone maleate) is an oral antidiabetic agent which acts primarily by increasing insulin sensitivity. AVANDIA is used in the management of type 2 diabetes mellitus (also known as non-insulin-dependent diabetes mellitus [NIDDM] or adult-onset diabetes). AVANDIA improves glycemic control while reducing circulating insulin levels. Pharmacological studies in animal models indicate that rosiglitazone improves sensitivity to insulin in muscle and adipose tissue and inhibits hepatic gluconeogenesis. Rosiglitazone maleate is not chemically or functionally related to the sulfonylureas, the biguanides, or the alpha-glucosidase inhibitors. Chemically, rosiglitazone maleate is (±)-5-[[4-[2-(methyl-2- pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione, (Z)-2-butenedioate (1:1) with a molecular weight of 473.52 (357.44 free base). The molecule has a single chiral center and is present as a racemate. Due to rapid interconversion, the enantiomers are functionally indistinguishable. The structural formula of rosiglitazone maleate is: The molecular formula is C18H19N3O3S•C4H4O4. -

Taking Metformin for Gestational Diabetes This Information Sheet Will Help You Understand More About Taking Metformin for Gestational Diabetes
Taking metformin for gestational diabetes This information sheet will help you understand more about taking metformin for gestational diabetes. This information sheet is not a substitute for talking with your midwife, doctor, nurse or pharmacist. Gestational diabetes Risks for your baby: Gestational diabetes means you have too much • may grow larger than normal (this increases the glucose (a type of sugar) in your blood when you risk of injury during birth) are pregnant. Insulin controls the amount of • born too early (premature) glucose in your blood. Sometimes you don’t make • low blood glucose after birth enough insulin or it doesn’t work as well when you are pregnant. • may need treatment in hospital • jaundice (yellow skin) that needs treatment Gestational diabetes is common. Up to eight pregnant women in every 100 get gestational • breathing problems. diabetes. Taking metformin helps reduce these risks. It can be hard to tell if you have gestational You can see how many women and babies are diabetes. Some women find that they: affected and how treatment helps in the picture • lack energy on the next page. • feel thirsty a lot • go to the toilet a lot Metformin is safe to take for • get lots of infections. gestational diabetes Metformin has been tested in clinical studies and Some women have no symptoms. This is why all is safe to take for gestational diabetes. pregnant women are tested for diabetes so they Metformin helps keep both your weight gain and can get treatment. blood pressure down. Metformin The main side effect is stomach upsets such as Metformin is a medicine that has been used feeling sick and diarrhoea.