A New Species of Anolis (Squamata: Iguanidae) from Panama
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
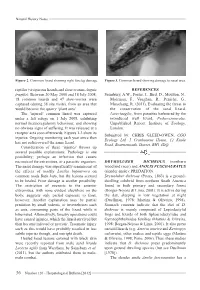
Reptiles (Viviparous Lizards and Slow-Worms Anguis REFERENCES Fragilis)
Natural History Notes Figure 2. Common lizard showing right foreleg damage. Figure 3. Common lizard showing damage to nasal area. reptiles (viviparous lizards and slow-worms Anguis REFERENCES fragilis). Between 20 May 2008 and 18 July 2008, Sainsbury, A.W., Foster, J., Bird, D., Moulton, N., 18 common lizards and 47 slow-worms were Molenaar, F., Vaughan, R., Peniche, G., captured (during 38 site visits), from an area that Marschang, R. (2011). Evaluating the threat to would become the quarry ‘plant area’. the conservation of the sand lizard, The ‘injured’ common lizard was captured Lacertaagilis, from parasites harboured by the under a felt refuge on 1 July 2008, exhibiting introduced wall lizard, Podarcismuralis. normal thermoregulatory behaviour, and showing Unpublished Report. Institute of Zoology, no obvious signs of suffering. It was released at a London. receptor area soon afterwards. Figures 1-3 show its Submitted by: CHRIS GLEED-OWEN, CGO injuries. Ongoing monitoring each year since then Ecology Ltd, 5 Cranbourne House, 12 Knole has not rediscovered the same lizard. Road, Bournemouth, Dorset, BH1 4DQ. Consideration of these ‘injuries’ throws up several possible explanations. Pathology is one possibility; perhaps an infection that causes necrosis of the extremities, or a parasitic organism. DRYMOLUBER DICHROUS (northern The nasal damage was superficially reminiscent of woodland racer) and ANOLIS FUSCOAURATUS the effects of toadfly Lucilia bufonivora on (slender anole): PREDATION. common toads Bufo bufo, but the lesions seemed Drymoluber dichrous (Peters, 1863) is a ground- to be healed. Frost damage is another possibility. dwelling colubrid from northern South America The restriction of necrosis to the anterior found in both primary and secondary forest extremities, with none evident elsewhere on the (Borges-Nojosa & Lima, 2001). -

Checklist of Helminths from Lizards and Amphisbaenians (Reptilia, Squamata) of South America Ticle R A
The Journal of Venomous Animals and Toxins including Tropical Diseases ISSN 1678-9199 | 2010 | volume 16 | issue 4 | pages 543-572 Checklist of helminths from lizards and amphisbaenians (Reptilia, Squamata) of South America TICLE R A Ávila RW (1), Silva RJ (1) EVIEW R (1) Department of Parasitology, Botucatu Biosciences Institute, São Paulo State University (UNESP – Univ Estadual Paulista), Botucatu, São Paulo State, Brazil. Abstract: A comprehensive and up to date summary of the literature on the helminth parasites of lizards and amphisbaenians from South America is herein presented. One-hundred eighteen lizard species from twelve countries were reported in the literature harboring a total of 155 helminth species, being none acanthocephalans, 15 cestodes, 20 trematodes and 111 nematodes. Of these, one record was from Chile and French Guiana, three from Colombia, three from Uruguay, eight from Bolivia, nine from Surinam, 13 from Paraguay, 12 from Venezuela, 27 from Ecuador, 17 from Argentina, 39 from Peru and 103 from Brazil. The present list provides host, geographical distribution (with the respective biome, when possible), site of infection and references from the parasites. A systematic parasite-host list is also provided. Key words: Cestoda, Nematoda, Trematoda, Squamata, neotropical. INTRODUCTION The present checklist summarizes the diversity of helminths from lizards and amphisbaenians Parasitological studies on helminths that of South America, providing a host-parasite list infect squamates (particularly lizards) in South with localities and biomes. America had recent increased in the past few years, with many new records of hosts and/or STUDIED REGIONS localities and description of several new species (1-3). -

Anolis Aeneus (Bronze Anole)
UWI The Online Guide to the Animals of Trinidad and Tobago Behaviour Anolis aeneus (Bronze Anole) Family: Polychrotidae (Anoles) Order: Squamata (Lizards and Snakes) Class: Reptilia (Reptiles) Fig. 1. Bronze anole, Anolis aeneus. [http://www.trinidad-tobagoherps.org/Anolisaeneus.htm, downloaded 20 October 2012] TRAITS. Anolis aeneus can be distinguished by the blue-green ring around its eyes (Murphy 2011). The species is a medium sized anole, the length of males from the tip of the nose to the anus is 77 mm and females 55 mm (John et al. 2012). They have many lamellae (flaps) on their subdigital toepads. The dewlap (throat fan) extends from underneath their necks and has a pale gray, green or white colour, yellow or orange spots may also be present at the front edge of the dewlap (John et al. 2012). Colour: The dorsal side of the males may be grey, greyish brown, brown or a dull green, a bronze sheen is at times present, light or dark spots may be present in a crosswise pattern. The underside has a dull grey colour. The females may be grey or brown the mid-dorsal region can include a dark single stripe or a transverse stripe, juveniles are dark grey or brown (John et al. 2012). ECOLOGY. This species is endemic to Grenada and has been introduced to Trinidad and Tobago (Wikipedia 2012). It is an arboreal species and can therefore be found mostly on the trunk and branches of shaded trees, it is also populated in urban areas and can be observed on walls, railings and fences (Murphy 2011) it feeds on live insects and invertebrates such as crickets, roaches and spiders. -

Anolis Planiceps (Leaf Anole)
UWI The Online Guide to the Animals of Trinidad and Tobago Diversity Anolis planiceps (Leaf Anole) Family: Polychrotidae (Anoles and Tree Lizards) Order: Squamata (Lizards and Snakes) Class: Reptilia (Reptiles) Fig. 1. Leaf anole, Anolis planiceps. [http://www.trinidad-tobagoherps.org/Images/planiceps.jpg, downloaded 24 October 2016] TRAITS. Formerly known as Anolis chrysolepis or Norops chrysolepis, the leaf anole measures up to 76mm from snout to vent according (D'Angiolella et al., 2011). The pads of their feet are specialised to help them rest on leaves and trunks (Fig. 1). They have a spotted red patch of skin below theirs jaws, which is extendable, called the dewlap (Fig. 2). The region along the lizard's spine has larger scales than the adjacent areas with those located in the mid-dorsal area being the largest. Along their heads are two prominent ridges as well as ridged (keeled) scales located above the eyes (Fig. 3). The dorsal scales of the leaf anole are several shades of brown while the ventral scales are a pale cream colour; patterns vary greatly within populations (Fig. 4) (Vanzolini and Williams, 1970). Male anoles have longer tails and the females have wider bodies and smaller dewlaps than males (Vitt and Zani, 2011). DISTRIBUTION. Leaf anoles may be found in a relatively wide range from east Venezuela to Guyana, Suriname, Columbia, Trinidad and Brazil (Fig. 5). They are found throughout the island of Trinidad primarily in terrestrial, highly forested areas (D'Angiolella et al, 2011). UWI The Online Guide to the Animals of Trinidad and Tobago Diversity HABITAT AND ECOLOGY. -

Diversificação Morfológica E Molecular Em Lagartos Dactyloidae Sul-Americanos
MUSEU PARAENSE EMÍLIO GOELDI UNIVERSIDADE FEDERAL DO PARÁ PROGRAMA DE PÓS-GRADUAÇÃO EM ZOOLOGIA CURSO DE DOUTORADO EM ZOOLOGIA DIVERSIFICAÇÃO MORFOLÓGICA E MOLECULAR EM LAGARTOS DACTYLOIDAE SUL-AMERICANOS ANNELISE BATISTA D’ANGIOLELLA Belém - PA 2015 ANNELISE BATISTA D’ANGIOLELLA DIVERSIFICAÇÃO MORFOLOGICA E MOLECULAR EM LAGARTOS DACTYLOIDAE SUL-AMERICANOS Tese apresenta ao Programa de Pós-Graduação em Zoologia do convênio Universidade Federal do Pará e Museu Paraense Emílio Goeldi, para obtenção do título de doutora em zoologia. Orientadora: Dra. Tereza Cristina Ávila Pires Co-Orientadora: Dra. Ana Carolina Carnaval Belém - PA 2015 “É capaz quem pensa que é capaz.” ii Agradecimento Ao CNPq pela concessão da minha bolsa de pesquisa. A Capes pela Bolsa de Doutorado Sanduiche no exterior. À Teresa Avila-Pires, minha orientadora, por estar sempre disponível para ajudar, escutar e puxar a orelha! A minha co-orientadora Carol Carnaval, por ter me recebido de braços abertos em seu lab e por toda confiança e apoio. A Ana Prudente pelo passe livre à Coleção e sugestões dadas ao trabalho de hemipenis. Ao Tibério Burlamaqui por toda a ajuda com as análises moleculares e momentos de descontração! A todo o pessoal do laboratório de Herpetologia do MPEG pela companhia e troca de ideias, sempre ajudando quando possível. Ao lab de molecular que foi a minha casa nesses últimos quatro anos e a todos que por ele passaram e contribuíram de alguma forma com meu conhecimento, em especial a Áurea, Geraldo, e Joice. Aos meus filhos de quatro patas Pukey e Bingo por me amarem incondicionalmente. A dança, por ser meu refúgio e por não ter me deixado pirar! Ao meu amor, Bruno, por me inspirar diariamente a ser uma pessoa melhor! Por me impulsionar a ir além e por simplesmente existir em minha vida.. -

Iguanidae: Hoplocercinae) from Southwestern Ecuador
Volume 48(20):227-235, 2008 A new species of ENYALIOIDES (Iguanidae: Hoplocercinae) from southwestern Ecuador Omar Torres-Carvajal1,2 Ana Almendáriz3 Jorge Valencia4 Mario Yánez-Muñoz5 Juan P. Reyes5 ABStract We describe a new species of Enyalioides from lowland cloud forests in southwestern Ecuador. This represents the third species in the genus known to occur west of the Andes in South America; the other two species are E. heterolepis and E. oshaughnessyi. Among other characters, the new species can be distinguished from other members in the genus by having small, keeled, paravertebrals; a series of skin folds on the lateral aspects of body and neck; size-homogeneous scales on body and limbs; distinct caudal segments; and an extensive dark patch on the gular region of adult males. Morphological similarity suggests that the new species, which we call E. touzeti, is closely related to E. oshaughnessyi. Keywords: Ecuador, Enyalioides, Hoplocercinae, Iguania, new species. INtroDUctioN Panama and southeastern Brazil, on both sides of the Andes, with most species occurring in Colombia, Ec- The neotropical iguanian lizard clade Hoplocer- uador, and Peru (Table 1). cidae (Frost & Etheridge, 1989; Frost et al., 2001), Despite the small size of this clade and its phylo- also known as Hoplocercinae (Macey et al., 1997; genetic importance as a possible basal lineage within Schulte et al., 2003), includes 11 species in three Iguania (Etheridge & de Queiroz, 1988; Schulte et al., genera (Enyalioides, Hoplocercus, and Morunasaurus). 1998, 2003), many questions remain to be answered. Hoplocercines are known from the lowlands between First, the phylogenetic relationships among its species 1. -

And Resurrection of Anolis (Diaphoranolis) Brooksi 1Steven Poe and 2Mason J
Ofcial journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 11(2) [General Section]: 1–16 (e141). urn:lsid:zoobank.org:pub:31FA8B4B-718B-4440-AE19-9E1AC95524BD Description of two new species similar to Anolis insignis (Squamata: Iguanidae) and resurrection of Anolis (Diaphoranolis) brooksi 1Steven Poe and 2Mason J. Ryan 1,3Department of Biology and Museum of Southwestern Biology, University of New Mexico, Albuquerque, New Mexico 87131, USA 2Arizona Game and Fish Department, 5000 W. Carefree Highway, Phoenix, AZ 85086, USA Abstract.—The spectacular giant anole lizard Anolis insignis is widely distributed but infrequently collected outside of northern Costa Rica. We recently collected several individuals similar to Anolis insignis from localities in Panama and southern Costa Rica. These populations difer from type locality A. insignis in male dewlap color and morphology. We associate one set of these populations with Anolis (Diaphoranolis) brooksi Barbour from Darién, Panama, and describe two additional populations as new species. Keywords. Central America, Costa Rica, lizard, Panama, Reptilia, taxonomy Citation: Poe S and Ryan MJ. 2017. Description of two new species similar to Anolis insignis (Squamata: Iguanidae) and resurrection of Anolis (Diaphoranolis) brooksi. Amphibian & Reptile Conservation 11(2) [General Section]: 1–16 (e141). Copyright: © 2017 Poe and Ryan. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International License, which permits unrestricted use for non-commercial and education purposes only, in any medium, provided the original author and the ofcial and authorized publication sources are recognized and properly credited. The ofcial and authorized publication credit sources, which will be duly enforced, are as follows: ofcial journal title Amphibian & Reptile Conservation; ofcial journal website <amphibian- reptile-conservation.org>. -

Multi-National Conservation of Alligator Lizards
MULTI-NATIONAL CONSERVATION OF ALLIGATOR LIZARDS: APPLIED SOCIOECOLOGICAL LESSONS FROM A FLAGSHIP GROUP by ADAM G. CLAUSE (Under the Direction of John Maerz) ABSTRACT The Anthropocene is defined by unprecedented human influence on the biosphere. Integrative conservation recognizes this inextricable coupling of human and natural systems, and mobilizes multiple epistemologies to seek equitable, enduring solutions to complex socioecological issues. Although a central motivation of global conservation practice is to protect at-risk species, such organisms may be the subject of competing social perspectives that can impede robust interventions. Furthermore, imperiled species are often chronically understudied, which prevents the immediate application of data-driven quantitative modeling approaches in conservation decision making. Instead, real-world management goals are regularly prioritized on the basis of expert opinion. Here, I explore how an organismal natural history perspective, when grounded in a critique of established human judgements, can help resolve socioecological conflicts and contextualize perceived threats related to threatened species conservation and policy development. To achieve this, I leverage a multi-national system anchored by a diverse, enigmatic, and often endangered New World clade: alligator lizards. Using a threat analysis and status assessment, I show that one recent petition to list a California alligator lizard, Elgaria panamintina, under the US Endangered Species Act often contradicts the best available science. -

Testing Sustainable Forestry Methods in Puerto Rico
Herpetology Notes, volume 8: 141-148 (2015) (published online on 10 April 2015) Testing sustainable forestry methods in Puerto Rico: Does the presence of the introduced timber tree Blue Mahoe, Talipariti elatum, affect the abundance of Anolis gundlachi? Norman Greenhawk Abstract. The island of Puerto Rico has one of the highest rates of regrowth of secondary forests largely due to abandonment of previously agricultural land. The study was aimed at determining the impact of the presence of Talipariti elatum, a timber species planted for forest enrichment, on the abundance of anoles at Las Casas de la Selva, a sustainable forestry project located in Patillas, Puerto Rico. The trees planted around 25 years ago are fast-growing and now dominate canopies where they were planted. Two areas, a control area of second-growth forest without T. elatum and an area within the T. elatum plantation, were surveyed over an 18 month period. The null hypothesis that anole abundance within the study areas is independent of the presence of T. elatum could not be rejected. The findings of this study may have implications when designing forest management practices where maintaining biodiversity is a goal. Keywords. Anolis gundlachi, Anolis stratulus, Puerto Rican herpetofauna, introduced species, forestry Introduction The secondary growth forest represents a significant resource base for the people of Puerto Rico, and, if At the time of Spanish colonization in 1508, nearly managed properly, an increase in suitable habitat one hundred percent of Puerto Rico was covered in for forest-dwelling herpetofauna. Depending on the forest (Wadsworth, 1950). As a result of forest clearing management methods used, human-altered agro- for agricultural and pastureland, ship building, and fuel forestry plantations have potential conservation wood, approximately one percent of the land surface value (Wunderle, 1999). -

Iguanid and Varanid CAMP 1992.Pdf
CONSERVATION ASSESSMENT AND MANAGEMENT PLAN FOR IGUANIDAE AND VARANIDAE WORKING DOCUMENT December 1994 Report from the workshop held 1-3 September 1992 Edited by Rick Hudson, Allison Alberts, Susie Ellis, Onnie Byers Compiled by the Workshop Participants A Collaborative Workshop AZA Lizard Taxon Advisory Group IUCN/SSC Conservation Breeding Specialist Group SPECIES SURVIVAL COMMISSION A Publication of the IUCN/SSC Conservation Breeding Specialist Group 12101 Johnny Cake Ridge Road, Apple Valley, MN 55124 USA A contribution of the IUCN/SSC Conservation Breeding Specialist Group, and the AZA Lizard Taxon Advisory Group. Cover Photo: Provided by Steve Reichling Hudson, R. A. Alberts, S. Ellis, 0. Byers. 1994. Conservation Assessment and Management Plan for lguanidae and Varanidae. IUCN/SSC Conservation Breeding Specialist Group: Apple Valley, MN. Additional copies of this publication can be ordered through the IUCN/SSC Conservation Breeding Specialist Group, 12101 Johnny Cake Ridge Road, Apple Valley, MN 55124. Send checks for US $35.00 (for printing and shipping costs) payable to CBSG; checks must be drawn on a US Banlc Funds may be wired to First Bank NA ABA No. 091000022, for credit to CBSG Account No. 1100 1210 1736. The work of the Conservation Breeding Specialist Group is made possible by generous contributions from the following members of the CBSG Institutional Conservation Council Conservators ($10,000 and above) Australasian Species Management Program Gladys Porter Zoo Arizona-Sonora Desert Museum Sponsors ($50-$249) Chicago Zoological -

(Squamata: Iguania) from the Central Andes of Colombia
HERPETOLOGICAL JOURNAL 20: 231–236, 2010 A new species of Anolis of the aequatorialis group (Squamata: Iguania) from the central Andes of Colombia Julián Andrés Velasco1, Paul David A. Gutiérrez-Cárdenas2 & Andrés Quintero-Angel1 1Grupo de Ecología Animal, Departamento de Biología, Universidad del Valle, Cali, Colombia 2Grupo Herpetológico de Antioquia (sede Caldas), Departamento de Ciencias Biológicas, Universidad de Caldas, Manizales, Colombia We describe a new species of the Anolis aequatorialis group from the central Andes of Colombia. The new species, Anolis anoriensis, is similar to A. eulaemus Boulenger, which occurs in both the western and central Andes, and was positioned in the eulaemus subgroup of the aequatorialis group. Anolis anoriensis differs from A. eulaemus in having smaller interparietal scales and a green body coloration with a darker anterior part of the dewlap. We also for the first time describe the coloration of Anolis eulaemus, which is almost exclusively brown with a diffused light brown dewlap. Key words: Anolis anoriensis sp. nov., taxonomy, morphology INTRODUCTION distributions in Colombia and Ecuador: A. antioquiae, A. eulaemus, A. fitchi, A. gemmosus, A. maculigula, A. meg- he Andes of Colombia are a recognized global biodi- alopithecus and A. ventrimaculatus. Tversity hotspot (Myers et al., 2000). However, many In this paper we describe a new species of Anolis of taxonomic groups have been poorly sampled in this re- the eulaemus subgroup of alpha anoles (Etheridge, 1959), gion, despite a large number of species discoveries in the from the Department of Antioquia in the Cordillera Cen- last decade. Anolis lizards are one of these poorly studied tral of Colombia. -

NAME: Land Iguana and Marine Iguana – Both in the Family Iguanidae CLASSIFICATION: Land: Ge- Nus Conolophus; Species (Two) Su
Grab & Go NAME: Land Iguana and Marine Iguana – both in the Family Iguanidae CLASSIFICATION: Land: Ge- nus Conolophus; Species (two) subcristatus and pallidus Marine: Genus Amblyrhynchus; Species cristatus MAIN MESSAGES: The Galapagos are a quintessential example of islands as living laboratories of evolution. ✤ Beginning with Darwin and Wallace, the study of islands has provided insight into how organisms colo- nize new environments and, through successive genera- tions, undergo changes that make their descendants more suited to thrive in the new environment CAS has made research ex- peditions to the Galapagos and has been involved in conservation efforts there for over 100 years. ✤ CAS collections from the Galapagos are the best in the world. Today, the Academy continues its research in the Galapagos and maintains the best collection of Galapagos materials in the world. Scientists at CAS collect plants and animals on research expedi- tions around the world. Their work and Academy collections provide invaluable baseline information about human impacts and change over time. DISTRIBUTION AND HABITAT: All are inhabitants of the Galapagos Islands. A spiny- tailed Central American Iguana may be ancestral to all three, but the marine species evolved from the terrestrial iguanas and thus is endemic to the Galapagos. Further, it is the only sea-going iguana in the world, and spends its entire life in the intertidal/sub- tidal zones. Although the land iguanas live on several islands, they are mostly seen on Plaza Sur. More widespread, the marine iguanas are seen in the channel between Isa- bella and Fernandina and along the cliffs of Espanola. DESCRIPTION AND DIET: Marine iguanas may range from 2 to 20 lbs, usually with a blackish color.