Baseline Surface Water Monitoring in Otsego County
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NON-TIDAL BENTHIC MONITORING DATABASE: Version 3.5
NON-TIDAL BENTHIC MONITORING DATABASE: Version 3.5 DATABASE DESIGN DOCUMENTATION AND DATA DICTIONARY 1 June 2013 Prepared for: United States Environmental Protection Agency Chesapeake Bay Program 410 Severn Avenue Annapolis, Maryland 21403 Prepared By: Interstate Commission on the Potomac River Basin 51 Monroe Street, PE-08 Rockville, Maryland 20850 Prepared for United States Environmental Protection Agency Chesapeake Bay Program 410 Severn Avenue Annapolis, MD 21403 By Jacqueline Johnson Interstate Commission on the Potomac River Basin To receive additional copies of the report please call or write: The Interstate Commission on the Potomac River Basin 51 Monroe Street, PE-08 Rockville, Maryland 20850 301-984-1908 Funds to support the document The Non-Tidal Benthic Monitoring Database: Version 3.0; Database Design Documentation And Data Dictionary was supported by the US Environmental Protection Agency Grant CB- CBxxxxxxxxxx-x Disclaimer The opinion expressed are those of the authors and should not be construed as representing the U.S. Government, the US Environmental Protection Agency, the several states or the signatories or Commissioners to the Interstate Commission on the Potomac River Basin: Maryland, Pennsylvania, Virginia, West Virginia or the District of Columbia. ii The Non-Tidal Benthic Monitoring Database: Version 3.5 TABLE OF CONTENTS BACKGROUND ................................................................................................................................................. 3 INTRODUCTION .............................................................................................................................................. -
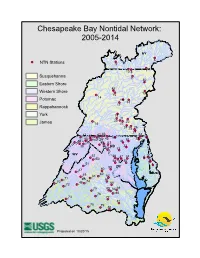
Chesapeake Bay Nontidal Network: 2005-2014
Chesapeake Bay Nontidal Network: 2005-2014 NY 6 NTN Stations 9 7 10 8 Susquehanna 11 82 Eastern Shore 83 Western Shore 12 15 14 Potomac 16 13 17 Rappahannock York 19 21 20 23 James 18 22 24 25 26 27 41 43 84 37 86 5 55 29 85 40 42 45 30 28 36 39 44 53 31 38 46 MD 32 54 33 WV 52 56 87 34 4 3 50 2 58 57 35 51 1 59 DC 47 60 62 DE 49 61 63 71 VA 67 70 48 74 68 72 75 65 64 69 76 66 73 77 81 78 79 80 Prepared on 10/20/15 Chesapeake Bay Nontidal Network: All Stations NTN Stations 91 NY 6 NTN New Stations 9 10 8 7 Susquehanna 11 82 Eastern Shore 83 12 Western Shore 92 15 16 Potomac 14 PA 13 Rappahannock 17 93 19 95 96 York 94 23 20 97 James 18 98 100 21 27 22 26 101 107 24 25 102 108 84 86 42 43 45 55 99 85 30 103 28 5 37 109 57 31 39 40 111 29 90 36 53 38 41 105 32 44 54 104 MD 106 WV 110 52 112 56 33 87 3 50 46 115 89 34 DC 4 51 2 59 58 114 47 60 35 1 DE 49 61 62 63 88 71 74 48 67 68 70 72 117 75 VA 64 69 116 76 65 66 73 77 81 78 79 80 Prepared on 10/20/15 Table 1. -

Susquehanna Riyer Drainage Basin
'M, General Hydrographic Water-Supply and Irrigation Paper No. 109 Series -j Investigations, 13 .N, Water Power, 9 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY CHARLES D. WALCOTT, DIRECTOR HYDROGRAPHY OF THE SUSQUEHANNA RIYER DRAINAGE BASIN BY JOHN C. HOYT AND ROBERT H. ANDERSON WASHINGTON GOVERNMENT PRINTING OFFICE 1 9 0 5 CONTENTS. Page. Letter of transmittaL_.__.______.____.__..__.___._______.._.__..__..__... 7 Introduction......---..-.-..-.--.-.-----............_-........--._.----.- 9 Acknowledgments -..___.______.._.___.________________.____.___--_----.. 9 Description of drainage area......--..--..--.....-_....-....-....-....--.- 10 General features- -----_.____._.__..__._.___._..__-____.__-__---------- 10 Susquehanna River below West Branch ___...______-_--__.------_.--. 19 Susquehanna River above West Branch .............................. 21 West Branch ....................................................... 23 Navigation .--..........._-..........-....................-...---..-....- 24 Measurements of flow..................-.....-..-.---......-.-..---...... 25 Susquehanna River at Binghamton, N. Y_-..---...-.-...----.....-..- 25 Ghenango River at Binghamton, N. Y................................ 34 Susquehanna River at Wilkesbarre, Pa......_............-...----_--. 43 Susquehanna River at Danville, Pa..........._..................._... 56 West Branch at Williamsport, Pa .._.................--...--....- _ - - 67 West Branch at Allenwood, Pa.....-........-...-.._.---.---.-..-.-.. 84 Juniata River at Newport, Pa...-----......--....-...-....--..-..---.- -

Otsego County Baseline Water Quality Monitoring1
1 Otsego County baseline water quality monitoring Scott Fickbohm2 INTRODUCTION The following is a preliminary report of water quality data collected between May 2009 and December 2010 at the outflow of Otsego County’s fourteen 11-digit Hydrologic Unit Codes watersheds (HUC’s). While extensive water quality monitoring is currently taking place in the County in specific waterbodies, this effort is meant to be a first step towards being able to characterize baseline water quality across Otsego County by means of direct measurement. Otsego County is 1,007 square miles in area. Estimates of land use are 71% forest, 27% in agriculture and 2% other (urban/developed). From the 11-digit HUC perspective, that area is divided between 14 distinct watersheds. The boundaries of these watersheds extend beyond the County borders and total an area of 1,390 square miles that all drain to the Susquehanna River and, ultimately, to the Chesapeake Bay. Approximately 27 square miles (5%) of Otsego County drains to the Mohawk River Basin through Canajoharie Creek and Cobleskill Creek. These Creeks were not sampled. An exception to the 11-digit HUC approach is the Butternut Creek & Lower Unadilla watersheds. At the 11 HUC level, the Butternut is limited to the area above Morris, NY with the lower portion being considered part of the Lower Unadilla watershed. In order to capture watershed specific data to the greatest extent possible, the Butternut was sampled just north of its confluence with the Unadilla River. The area for each of these watersheds was recalculated based on this sampling point. The names of each watershed sampled, along with their HUC number and area, are provided in the Table 1. -

Otsego County Water Quality Coordinating Committee Annual Report 2010 & 2011
Otsego County Water Quality Coordinating Committee Annual Report 2010 & 2011 Prepared by: Otsego County Water Quality Coordinating Committee 967 County Highway 33 Cooperstown, NY 13326 6/18/2012 Table of Contents INTRODUCTION OF WATER QUALITY COORDINATING COMMITTEE 2 COORDINATING COMMITTEE MEMBERS 2 COMMITTEE MISSION, PURPOSE AND PRIMARY FUNCTIONS 3 2010 ACCOMPLISHMENTS 5 2011 ACCOMPLISHMENTS 7 ATTACHMENTS: #1 Water Quality Coordinating Committee By Laws #2 2011 Otsego County Nonpoint Source Water Quality Strategy #3 Susquehanna Headwaters Nutrient Report #4 Otsego County Soil & Water Conservation District 2011 Report #5 Otsego County Conservation Association 2011 Report #6 Goodyear Lake Association 2011 Report #7 Otsego Lake Watershed Supervisory Committee 2011 Report #8 SUNY Oneonta Biological Field Station 2011 Staff Activity Report #9 Village of Richfield 2011 Water Quality Report 1 INTRODUCTION OTSEGO COUNTY WATER QUALITY COORDINATING COMMITTEE Non-point pollution (NPS) by definition is any form of pollution not being discharged from a distinguished point or source. Sources of NPS, being so diffuse and variable, will often require multi-agency involvement to remediate the existing water pollution problems. Understanding that the responsibility and interest in water quality issues are represented by a wide range of parties, a committee was formed and is known as the Otsego County Water Quality Coordinating Committee (the Committee) and functions as a subcommittee of the Otsego County Soil and Water Conservation District. The Committee is responsible for the preparation of the Otsego County Non-point Source Water Quality Strategy. The Strategy attempts to ensure that the individual efforts of local, county, state, and federal agencies regarding water quality programs and educational outreach events are coordinated for maximum effectiveness. -

Public Fishing Rights Maps: Otego Creek
Public Fishing Rights Maps Otego Creek Fish Species Present About Public Fishing Rights Brown Trout Public Fishing Rights (PFR’s) are permanent easements purchased by the NYSDEC from will- ing landowners, giving anglers the right to fish and walk along the bank (usually a 33’ strip on Brook Trout one or both banks of the stream). This right is for the purpose of fishing only and no other purpose. Treat the land with respect to insure the continua- tion of this right and privilege. Fishing privileges may be available on some other private lands with Description of Fishery permission of the land owner. Courtesy toward the land-owner and respect for their property will Otego Creek flows for 29 miles before entering the insure their continued use. Susquehanna River west of Oneonta. The lower 10 miles supports a low quality warmwater fish- These generalized location maps are intended to ery for walleye, smallmouth bass, and rock bass. aid anglers in finding PFR segments and are not From Laurens upstream to Hartwick, the wild trout survey quality. Width of displayed PFR may be population in this 14.6 mile reach is supplemented wider than reality to make it more visible on the with the stocking of approximately 2,900 yearling maps. Please look for this PFR sign to ensure that and 125 two year old brown trout annually. Brown you are in the right location and have legal access trout abundance is higher in the lower reach and to the stream bank. brook trout in the upper reach. Many of the tribu- taries to Otego Creek are dominated by wild brook trout. -

Upper Susquehanna Subbasin Survey: a Water Quality and Biological Assessment, June – September 2007
Upper Susquehanna Subbasin Survey: A Water Quality and Biological Assessment, June – September 2007 The Susquehanna River Basin Commission (SRBC) conducted a water quality and biological survey of the Upper Susquehanna Subbasin from June to September 2007. This survey is part of SRBC’s Subbasin Survey Program, which is funded in part by the United States Environmental Protection Agency (USEPA). The Subbasin Survey Program consists of two- year assessments in each of the six major subbasins (Figure 1) on a rotating schedule. This report details the Year-1 survey, which consists of point-in-time water chemistry, macroinvertebrate, and habitat data collection and assessments of the major tributaries and areas of interest throughout the Upper Susquehanna Subbasin. The Year-2 survey will be conducted in the Tioughnioga River over a one-year time period beginning in summer 2008. The Year-2 survey is part of a larger monitoring effort associated with an environmental restoration effort at Whitney Point Lake. Previous SRBC surveys of the Upper Susquehanna Subbasin were conducted in 1998 (Stoe, 1999) and 1984 (McMorran, 1985). Subbasin survey information is used by SRBC staff and others to: • evaluate the chemical, biological, and habitat conditions of streams in the basin; • identify major sources of pollution and lengths of streams impacted; • identify high quality sections of streams that need to be protected; • maintain a database that can be used to document changes in stream quality over time; • review projects affecting water quality in the basin; and • identify areas for more intensive study. Description of the Upper Susquehanna Subbasin The Upper Susquehanna Subbasin is an interstate subbasin that drains approximately 4,950 square miles of southcentral New York and a small portion of northeastern Pennsylvania. -
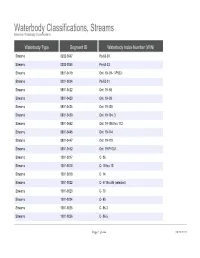
Waterbody Classifications, Streams Based on Waterbody Classifications
Waterbody Classifications, Streams Based on Waterbody Classifications Waterbody Type Segment ID Waterbody Index Number (WIN) Streams 0202-0047 Pa-63-30 Streams 0202-0048 Pa-63-33 Streams 0801-0419 Ont 19- 94- 1-P922- Streams 0201-0034 Pa-53-21 Streams 0801-0422 Ont 19- 98 Streams 0801-0423 Ont 19- 99 Streams 0801-0424 Ont 19-103 Streams 0801-0429 Ont 19-104- 3 Streams 0801-0442 Ont 19-105 thru 112 Streams 0801-0445 Ont 19-114 Streams 0801-0447 Ont 19-119 Streams 0801-0452 Ont 19-P1007- Streams 1001-0017 C- 86 Streams 1001-0018 C- 5 thru 13 Streams 1001-0019 C- 14 Streams 1001-0022 C- 57 thru 95 (selected) Streams 1001-0023 C- 73 Streams 1001-0024 C- 80 Streams 1001-0025 C- 86-3 Streams 1001-0026 C- 86-5 Page 1 of 464 09/28/2021 Waterbody Classifications, Streams Based on Waterbody Classifications Name Description Clear Creek and tribs entire stream and tribs Mud Creek and tribs entire stream and tribs Tribs to Long Lake total length of all tribs to lake Little Valley Creek, Upper, and tribs stream and tribs, above Elkdale Kents Creek and tribs entire stream and tribs Crystal Creek, Upper, and tribs stream and tribs, above Forestport Alder Creek and tribs entire stream and tribs Bear Creek and tribs entire stream and tribs Minor Tribs to Kayuta Lake total length of select tribs to the lake Little Black Creek, Upper, and tribs stream and tribs, above Wheelertown Twin Lakes Stream and tribs entire stream and tribs Tribs to North Lake total length of all tribs to lake Mill Brook and minor tribs entire stream and selected tribs Riley Brook -

Brook Trout Outcome Management Strategy
Brook Trout Outcome Management Strategy Introduction Brook Trout symbolize healthy waters because they rely on clean, cold stream habitat and are sensitive to rising stream temperatures, thereby serving as an aquatic version of a “canary in a coal mine”. Brook Trout are also highly prized by recreational anglers and have been designated as the state fish in many eastern states. They are an essential part of the headwater stream ecosystem, an important part of the upper watershed’s natural heritage and a valuable recreational resource. Land trusts in West Virginia, New York and Virginia have found that the possibility of restoring Brook Trout to local streams can act as a motivator for private landowners to take conservation actions, whether it is installing a fence that will exclude livestock from a waterway or putting their land under a conservation easement. The decline of Brook Trout serves as a warning about the health of local waterways and the lands draining to them. More than a century of declining Brook Trout populations has led to lost economic revenue and recreational fishing opportunities in the Bay’s headwaters. Chesapeake Bay Management Strategy: Brook Trout March 16, 2015 - DRAFT I. Goal, Outcome and Baseline This management strategy identifies approaches for achieving the following goal and outcome: Vital Habitats Goal: Restore, enhance and protect a network of land and water habitats to support fish and wildlife, and to afford other public benefits, including water quality, recreational uses and scenic value across the watershed. Brook Trout Outcome: Restore and sustain naturally reproducing Brook Trout populations in Chesapeake Bay headwater streams, with an eight percent increase in occupied habitat by 2025. -

Summary of Nitrogen, Phosphorus, and Suspended-Sediment Loads and Trends Measured at the Chesapeake Bay Nontidal Network Stations for Water Years 2009–2018
Summary of Nitrogen, Phosphorus, and Suspended-Sediment Loads and Trends Measured at the Chesapeake Bay Nontidal Network Stations for Water Years 2009–2018 Prepared by Douglas L. Moyer and Joel D. Blomquist, U.S. Geological Survey, March 2, 2020 The Chesapeake Bay nontidal network (NTN) currently consists of 123 stations throughout the Chesapeake Bay watershed. Stations are located near U.S. Geological Survey (USGS) stream-flow gages to permit estimates of nutrient and sediment loadings and trends in the amount of loadings delivered downstream. Routine samples are collected monthly, and 8 additional storm-event samples are also collected to obtain a total of 20 samples per year, representing a range of discharge and loading conditions (Chesapeake Bay Program, 2020). The Chesapeake Bay partnership uses results from this monitoring network to focus restoration strategies and track progress in restoring the Chesapeake Bay. Methods Changes in nitrogen, phosphorus, and suspended-sediment loads in rivers across the Chesapeake Bay watershed have been calculated using monitoring data from 123 NTN stations (Moyer and Langland, 2020). Constituent loads are calculated with at least 5 years of monitoring data, and trends are reported after at least 10 years of data collection. Additional information for each monitoring station is available through the USGS website “Water-Quality Loads and Trends at Nontidal Monitoring Stations in the Chesapeake Bay Watershed” (https://cbrim.er.usgs.gov/). This website provides State, Federal, and local partners as well as the general public ready access to a wide range of data for nutrient and sediment conditions across the Chesapeake Bay watershed. In this summary, results are reported for the 10-year period from 2009 through 2018. -
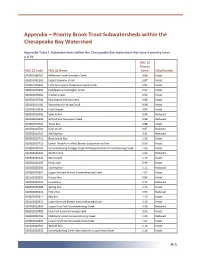
Appendix – Priority Brook Trout Subwatersheds Within the Chesapeake Bay Watershed
Appendix – Priority Brook Trout Subwatersheds within the Chesapeake Bay Watershed Appendix Table I. Subwatersheds within the Chesapeake Bay watershed that have a priority score ≥ 0.79. HUC 12 Priority HUC 12 Code HUC 12 Name Score Classification 020501060202 Millstone Creek-Schrader Creek 0.86 Intact 020501061302 Upper Bowman Creek 0.87 Intact 020501070401 Little Nescopeck Creek-Nescopeck Creek 0.83 Intact 020501070501 Headwaters Huntington Creek 0.97 Intact 020501070502 Kitchen Creek 0.92 Intact 020501070701 East Branch Fishing Creek 0.86 Intact 020501070702 West Branch Fishing Creek 0.98 Intact 020502010504 Cold Stream 0.89 Intact 020502010505 Sixmile Run 0.94 Reduced 020502010602 Gifford Run-Mosquito Creek 0.88 Reduced 020502010702 Trout Run 0.88 Intact 020502010704 Deer Creek 0.87 Reduced 020502010710 Sterling Run 0.91 Reduced 020502010711 Birch Island Run 1.24 Intact 020502010712 Lower Three Runs-West Branch Susquehanna River 0.99 Intact 020502020102 Sinnemahoning Portage Creek-Driftwood Branch Sinnemahoning Creek 1.03 Intact 020502020203 North Creek 1.06 Reduced 020502020204 West Creek 1.19 Intact 020502020205 Hunts Run 0.99 Intact 020502020206 Sterling Run 1.15 Reduced 020502020301 Upper Bennett Branch Sinnemahoning Creek 1.07 Intact 020502020302 Kersey Run 0.84 Intact 020502020303 Laurel Run 0.93 Reduced 020502020306 Spring Run 1.13 Intact 020502020310 Hicks Run 0.94 Reduced 020502020311 Mix Run 1.19 Intact 020502020312 Lower Bennett Branch Sinnemahoning Creek 1.13 Intact 020502020403 Upper First Fork Sinnemahoning Creek 0.96 -

On the Formation of Fluvial Islands
AN ABSTRACT OF THE DISSERTATION OF Joshua R. Wyrick for the degree of Doctor of Philosophy in Civil Engineering presented on March 31, 2005. Title: On the Formation of Fluvial Islands Abstract approved: Signature redacted for privacy. Peter C. Klingeman This research analyzes the effects of islands on river process and the effects river processes have on island formation. A fluvial island is defined herein as a land mass within a river channel that is separated from the floodplain by water on all sides, exhibits some stability, and remains exposed during bankfull flow. Fluvial islands are present in nearly all major rivers. They must therefore have some impact on the fluid mechanics of the system, and yet there has never been a detailed study on fluvial islands.Islands represent a more natural state of a river system and have been shown to provide hydrologic variability and biotic diversity for the river. This research describes the formation of fluvial islands, investigates the formation of fluvial islands experimentally, determines the main relations between fluvial islands and river processes, compares and describes relationships between fluvial islands and residual islands found in megaflood outwash plains, and reaches conclusions regarding island shape evolution and flow energy loss optimization. Fluvial islands are known to form by at least nine separate processes: avulsion, gradual degradation of channel branches, lateral shifts in channel position, stabilization of a bar or riffle, isolation of structural features, rapid incision of flood deposits, sediment deposition in the lee of an obstacle, isolation of material deposited by mass movement, and isolation of riparian topography after the installation of a dam.