Daidzein and Genistein Contents of Vegetables
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Note High-Performance Liquid Chromatography
Journal of Chromatography. 234 (1982) 494--496 Elsevier Scientific Publishing Company, Amsterdam - Printed in The Netherlands CHROM. 14.241 Note High-performance liquid chromatography separation of soybean iso flavones and their glucosides A. C. ELDRIDGE Nonhern Regional Research Celller. Agricll/Illra/ Research. Science and Edllcmion Administration, U.S. Department of Agricll/llIre, 1815 North University Street, Peoria. 1L 61604 (U.S.A.; (Received July 27th, 1981) Soybeans are known to contain several isoflavones (daidzein, glycitein, genis tein) and isoflavone glucosides l (daidzin, glycitein-7-f3-0-glucoside, genistin) which 2 3 4 have been reported to have estrogenic , antifungal and antioxidant activity. The quantitative determination of soybean isoflavones has been reported by gas-liquid chromatography (GLC)l, and more recently high-performance liquid chromatogra phy (HPLC)5.6.7 has been used. Carlson and Dolphin5 separated soybean isoflavone aglycones present in an alcohol extract on a pPorasil column. The isoflavone glucosides were determined after hydrolytic treatment with aqueous acid. In a paper by West et al. 6 only two isoflavone aglycones from soybean extracts were separated. This communication reports the development of a procedure for the separation of the naturally occurring soybean isoflavone glucosides and isoflavone algycones using HPLC with mild solvents and reversed phase packing that may be less likely to catalyze decomposition compared to other packings. MATERIALS AND METHODS* A Waters Assoc. (Milford, MA, U.S.A.) HPLC system was used, comprised of a WISP 710A, M-45 solvent delivery system, Model 660 solvent flow programmer, Model 450 variable-wavelength detector, with a DuPont Zorbax ODS 25 x 0.46 cm 1.0. -

Study Protocol and Statistical Analysis Plan
Confidential Clinical study protocol number: J1228 Page 1 Version Date: May 7, 2018 IRB study Number: NA_00067315 A Trial of maintenance Rituximab with mTor inhibition after High-dose Consolidative Therapy in CD20+, B-cell Lymphomas, Gray Zone Lymphoma, and Hodgkin’s Lymphoma Principal Investigator: Douglas E. Gladstone, MD The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins 1650 Orleans Street, CRBI-287 Baltimore, MD 21287 Phone: 410-955-8781 Fax: 410-614-1005 Email: [email protected] IRB Protocol Number: NA_00067315 Study Number: J1228 IND Number: EXEMPT Novartis Protocol Number: CRAD001NUS157T Version: May 7, 2018 Co-Investigators: Jonathan Powell 1650 Orleans Street, CRBI-443 Phone: 410-502-7887 Fax: 443-287-4653 Email: [email protected] Richard Jones 1650 Orleans Street, CRBI-244 Phone: 410-955-2006 Fax: 410-614-7279 Email: [email protected] Confidential Clinical study protocol number: J1228 Page 2 Version Date: May 7, 2018 IRB study Number: NA_00067315 Satish Shanbhag Johns Hopkins Bayview Medical Center 301 Building, Suite 4500 4940 Eastern Ave Phone: 410-550-4061 Fax: 410-550-5445 Email: [email protected] Statisticians: Gary Rosner Phone: 410-955-4884 Email: [email protected] Marianna Zahurak Phone: 410-955-4219 Email: [email protected] Confidential Clinical study protocol number: J1228 Page 3 Version Date: May 7, 2018 IRB study Number: NA_00067315 Table of contents Table of contents ......................................................................................................................... 3 List of abbreviations -
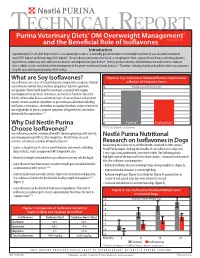
What Are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy Isoflavones Are a Class of Natural Bioactive Compounds in Soybeans
TECHNICAL REPORT Purina Veterinary Diets® OM Overweight Management® brand canine dry formula and the Beneficial Role of Isoflavones Introduction Approximately 35% of adult dogs in the U.S. are overweight or obese1. A markedly greater incidence of overweight and obesity was observed in neutered male (59% higher) and female dogs (40% higher)1. Chronic obesity can increase the risk of, or complications from, several chronic diseases including diabetes, hypertension, pulmonary and cardiovascular disease, and degenerative joint disease2. Obesity produces chronic, mild inflammation and increases oxidative stress, which, in turn, contributes to the development of the above-mentioned chronic diseases.3,4 Therefore, reducing obesity and oxidative stress can promote a long life span and improved quality of life in dogs. What are Soy Isoflavones? Figure 2: Soy Isoflavones Reduced Plasma Isoprostanes Soy isoflavones are a class of natural bioactive compounds in soybeans. Natural a Marker of Oxidative Stress soy isoflavones include three chemical compounds: daidzein, genistein, 5 Plasma Isoprostanes (ng/ml) and glycitein. Many health benefits have been associated with regular consumption of soy products. In humans, soy has been found to reduce the 4 risk of cardiovascular disease and certain types of cancer (breast and prostate cancer); relieve a number of problems in post menopausal women including 3 hot flashes, osteoporosis, and decline in cognitive function; reduce cholesterol and triglycerides in plasma; improve symptoms of hypertension; and reduce 2 abdominal fat accumulation.5,6,7 1 Why Did Nestlé Purina 0 Control Isoflavones* Choose Isoflavones? *P<0.01 for Control vs. Isoflavones Soy isoflavones provide a number of benefits for managing dogs with obesity, and reducing rebound effects after weight loss. -

Changes of Phytoestrogens Daidzein, Genistein and Their Glycosides Daidzin and Genistin and Coumestrol During Processing of Soyabeans
Czech J. Food Sci. Vol. 22, Special Issue Changes of Phytoestrogens Daidzein, Genistein and Their Glycosides Daidzin and Genistin and Coumestrol during Processing of Soyabeans J. LOJZA*, V. SCHULZOVÁ and J. HAJŠLOVÁ Department of Food Chemistry and Analysis, Institute of Chemical Technology, Prague, Czech Republic, *E-mail: [email protected] Abstract: Phytoestrogens represent biologically active compounds showing estrogenic activity similar to that of sex hormones – estrogens. Various adverse effects such as sterility, increase of females’ genitals, lost of males’ copulation activity, etc. were observed in farm animals after exposure to higher amounts of fodder containing phytoestrogens. On the other side, their presence in human diet is nowadays the object of many research stud- ies concerned with prevention of breast and prostate cancer, osteoporosis and other hormone-linked diseases by dietary intake of phytoestrogens. Soya (Glycine max) is one of the main sources of these compounds in diet. Isoflavones daidzein and genistein occurring either free or bound in glycosides are the main phytoestrogens in this food crop. Coumesterol representing coumestans is another effective phytoestrogen contained in some ed- dible plants. In the first part of our study, analytical method for determination of free and total phytoestrogens was developed and validated. Following steps are included: (i) acid hydrolysis (only for “total phytoestrogens” analysis), (ii) extraction with methanol/water mixture, (iii) SPE preconcentration; (iv) identification/quantifica- tion using HPLC/DAD/FLD. The aim of present study was to document the fate of phytoestrogens and their forms during household/industrial processing. As documented in our experiments the most dynamic changes of phytoestrogen levels occur during soyabeans sprouting. -

Antiproliferative Effects of Isoflavones on Human Cancer Cell Lines Established from the Gastrointestinal Tract 1
[CANCER RESEARCH 53, 5815-5821, December 1, 1993] Antiproliferative Effects of Isoflavones on Human Cancer Cell Lines Established from the Gastrointestinal Tract 1 Kazuyoshi Yanagihara, 2 Akihiro Ito, Tetsuya Toge, and Michitaka Numoto Department of Pathology [K. Y, M. N.], Cancer Research [A. L], and Surgery iT. T.], Research Institute for Nuclear Medicine and Biology, Hiroshima University, Kasumi 1-2-3, Minami-ku, Hiroshima 734, Japan ABSTRACT MATERIALS AND METHODS Seven isoflavones, biochanin A, daidzein, genistein, genistin, prunectin, Establishment of Cancer Cell Lines. Tumor tissues were trimmed of fat puerarin, and pseudobaptigenin were tested for cytostatic and cytotoxic and necrotic portions and minced with scalpels. The tissue pieces were trans- effects on 10 newly established cancer cell lines of the human gastrointes- ferred, together with medium, at 10 to 15 fragments/dish to 60-mm culture tinal origin. Proliferation of HSC-41E6, HSC-45M2, and SH101-P4 stom- dishes (Falcon, Lincoln Park, N J). The patient's ascitic tumor cells were ach cancer cell lines was strongly inhibited by biochanin A and genistein, harvested by centrifugation (1000 rpm for 10 min) and plated into 60-mm whereas other stomach, esophageal, and colon cancer lines were moder- dishes. Culture dishes initially selectively trypsinized [trypsin, 0.05% (w/v); ately suppressed by both compounds. Biochanin A and genistein were EDTA, 0.02% (w/v)], to remove overgrowing fibroblasts. In addition, we cytostatic at low concentrations (<20 pg/ml for biochanin A, <10/tg/ml for attempted to remove the fibroblasts mechanically and to transfer the tumor genistein) and were cytotoxic at higher concentrations (>40 pg/ml for cells selectively. -

Applications of in Silico Methods to Analyze the Toxicity and Estrogen T Receptor-Mediated Properties of Plant-Derived Phytochemicals ∗ K
Food and Chemical Toxicology 125 (2019) 361–369 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Applications of in silico methods to analyze the toxicity and estrogen T receptor-mediated properties of plant-derived phytochemicals ∗ K. Kranthi Kumara, P. Yugandharb, B. Uma Devia, T. Siva Kumara, N. Savithrammab, P. Neerajaa, a Department of Zoology, Sri Venkateswara University, Tirupati, 517502, India b Department of Botany, Sri Venkateswara University, Tirupati, 517502, India ARTICLE INFO ABSTRACT Keywords: A myriad of phytochemicals may have potential to lead toxicity and endocrine disruption effects by interfering Phytochemicals with nuclear hormone receptors. In this examination, the toxicity and estrogen receptor−binding abilities of a QSAR modeling set of 2826 phytochemicals were evaluated. The endpoints mutagenicity, carcinogenicity (both CAESAR and ISS Toxicity models), developmental toxicity, skin sensitization and estrogen receptor relative binding affinity (ER_RBA) Nuclear hormone receptor binding were studied using the VEGA QSAR modeling package. Alongside the predictions, models were providing pos- Self−Organizing maps sible information for applicability domains and most similar compounds as similarity sets from their training Clustering and classification schemes sets. This information was subjected to perform the clustering and classification of chemicals using Self−Organizing Maps. The identified clusters and their respective indicators were considered as potential hotspot structures for the specified data set analysis. Molecular screening interpretations of models wereex- hibited accurate predictions. Moreover, the indication sets were defined significant clusters and cluster in- dicators with probable prediction labels (precision). Accordingly, developed QSAR models showed good pre- dictive abilities and robustness, which observed from applicability domains, representation spaces, clustering and classification schemes. -

Daidzein and Genistein Content of Cereals
European Journal of Clinical Nutrition (2002) 56, 961–966 ß 2002 Nature Publishing Group All rights reserved 0954–3007/02 $25.00 www.nature.com/ejcn ORIGINAL COMMUNICATION Daidzein and genistein content of cereals J Liggins1, A Mulligan1,2, S Runswick1 and SA Bingham1,2* 1Medical Research Council Dunn Human Nutrition Unit, Hills Road, Cambridge, UK; and 2European Prospective Investigation of Cancer, University of Cambridge, Cambridge, UK Objective: To analyse 75 cereals and three soy flours commonly eaten in Europe for the phytoestrogens daidzein and genistein. Design: The phytoestrogens daidzein and genistein were extracted from dried foods, and the two isoflavones quantified after hydrolytic removal of any conjugated carbohydrate. Completeness of extraction and any procedural losses of the isoflavones 0 0 were accounted for using synthetic daidzin (7-O-glucosyl-4 -hydroxyisoflavone) and genistin (7-O-glucosyl-4 5-dihydroxyiso- flavone) as internal standards. Setting: Foods from the Cambridge UK area were purchased, prepared for eating, which included cooking if necessary, and freeze dried. Three stock soy flours were also analysed. Results: Eighteen of the foods assayed contained trace or no detectable daidzein or genistein. The soy flours were rich sources, containing 1639 – 2117 mg=kg. The concentration of the two isoflavones in the remaining foods ranged from 33 to 11 873 mg=kg. Conclusion: These analyses will supply useful information to investigators determining the intake of phytoestrogens in cereal products in order to relate intakes to potential biological activities. Sponsorship: This work was supported by the United Kingdom Medical Research Council, Ministry of Agriculture Fisheries and Food (contract FS2034) and the United States of America Army (contract DAMD 17-97-1-7028). -

Mindy Goldman, MD Clinical Professor Dept
Managing Menopause Medically and Naturally Mindy Goldman, MD Clinical Professor Dept. of Ob/Gyn and Reproductive Sciences Director, Women’s Cancer Care Program, UCSF Breast Care Center and Women’s Health University of California, San Francisco I have nothing to disclose –Mindy Goldman, MD CASE STUDY 50 yr. old G2P2 peri-menopausal woman presents with complaints of significant night sweats interfering with her ability to sleep. She has mild hot flashes during the day. She has never had a bone mineral density test but her mother had a hip fracture at age 62 due to osteoporosis. Her 46 yr. old sister was diagnosed with breast cancer at age 43, treated with lumpectomy and radiation and currently is doing well. There is no other family history of cancer. Questions 1. Would you offer her MHT? 2. If yes, how long would you continue it? 3. If no, what would you offer for alternative treatments? 4. Would your treatment differ if you knew she had underlying heart disease? Is it safe? How long can I take it? What about Mymy Bones?bones? Will it protect my heart? MHT - 2015 What about my brain? Will I get breast cancer? What about my hot flashes? Menopausal Symptoms Hot flashes Night sweats Sleep disturbances Vaginal dryness/Sexual dysfunction Mood disturbances How to Treat Menopausal Symptoms Hormone therapy Alternatives to hormones Complementary and Integrative Techniques Prior to Women’s Health Initiative Hormone therapy primary treatment of menopausal hot flashes Few women would continue hormones past one year By 1990’s well known -

Simultaneous Determination of Daidzein, Genistein and Formononetin in Coffee by Capillary Zone Electrophoresis
separations Article Simultaneous Determination of Daidzein, Genistein and Formononetin in Coffee by Capillary Zone Electrophoresis Feng Luan *, Li Li Tang, Xuan Xuan Chen and Hui Tao Liu College of Chemistry and Chemical Engineering, Yantai University, Yantai 264005, China; [email protected] (L.L.T.); [email protected] (X.X.C.); [email protected] (H.T.L.) * Correspondence: fl[email protected]; Tel.: +86-535-6902063 Academic Editor: Doo Soo Chung Received: 29 October 2016; Accepted: 20 December 2016; Published: 1 January 2017 Abstract: Coffee is a favorite and beverage in Western countries that is consumed daily. In the present study, capillary zone electrophoresis (CE) was applied for the separation and quantification of three isoflavones including daidzein, genistein and formononetin in coffee. Extraction of isoflavones from the coffee sample was carried out by extraction and purification process using ether after the acid hydrolysis with the antioxidant butylated hydroxy-toluene (BHT). The experimental conditions of the CE separation method were: 20 mmol/L Na2HPO4 buffer solution, 25 kV applied voltage, 3 s hydrodynamic injection at 30 mbar, and UV detection at 254 nm. The results show that the three compounds can be tested within 10 min with a linearity of 0.5–50 µg/mL for all three compounds. The limits of detection were 0.0642, 0.134, and 0.0825 µg/mL for daidzein, formononetin and genistein, respectively. The corresponding average recovery was 99.39% (Relative Standard Detection (RSD) = 1.76%), 98.71% (RSD = 2.11%) and 97.37% (RSD = 3.74%). Keywords: capillary zone electrophoresis (CE); daidzein; genistein; formononetin; acid hydrolysis 1. -

Contemporary Approaches for Managing Menopause Symptoms
Menopause 2017 Guidelines: Looking at Special Populations and Building on Existing Practice Patricia Geraghty, MSN, FNP-BC, WHNP Disclosures • Speaker Bureau • AbbVie • Therapeutics MD • Advisory Board • AbbVie (endometriosis) • Sharecare Inc. • Procedure Proctor • Bayer (Contraception) • Off label discussion will be included and identified in this discussion. Objectives • Identify the benefits and risks of estrogen, progesterone, and non-hormonal pharmacological management of menopause symptoms based on dosage, route of administration, pharmacokinetics, treatment population and duration of use. • Develop treatment strategies for special populations including premature ovarian insufficiency, prolonged symptoms, and women who are not candidates for estrogen. • Incorporate the NAMS 2017 Position Statement into patient counseling on the timeline of menopause symptoms and the termination of hormone therapy. Lifespan Lifespan Median 34 322 BC Aristotle describes transition Ages the Through Menopause yr JAMA. JAMA. Investigators. Initiative Health the Women's for Group Writing 2002;288(3):321 - 333. doi:10.1001/jama.288.3.321 Treated with plants s/a 1800’s cohosh, cannabis, opium 1821 French physician DeGardanne 53 lifespan lifespan calls it “Menopause” Adult - 1900’s Deficiency disease 55 1942 Premarin” copyright yr 1960’s “Forever Feminine” by Robert Wilson MD 1980’s-1990’s “Politics of Menopause” by Frances McCrea 2002 WHI- First large randomized control trial “My periods are different. Is This Menopause?” • Random cycling, sometimes early, sometimes late. • Flow variable with prodromal spotting, a long taper, or stopping and starting. • She has a day of very heavy flow. • 77% have duration 10+ days, heavy bleeding 3+ days, spotting 6+ days Paramsothy P, et al. BJOG. 2014 Nov;121(12):1564-73. -

Soy Isoflavone Genistein Inhibits an Axillary Osmidrosis Risk Factor ABCC11: in Vitro Screening and Fractional Approach for ABCC11-Inhibitory Activities in Plant Extracts and Dietary
nutrients Article Soy Isoflavone Genistein Inhibits an Axillary Osmidrosis Risk Factor ABCC11: In Vitro Screening and Fractional Approach for ABCC11-Inhibitory Activities in Plant Extracts and Dietary Flavonoids 1,2, 2, , 1 1 1 Hiroki Saito y, Yu Toyoda * y , Hiroshi Hirata , Ami Ota-Kontani , Youichi Tsuchiya , Tappei Takada 2 and Hiroshi Suzuki 2 1 Frontier Laboratories for Value Creation, Sapporo Holdings Ltd., 10 Okatome, Yaizu, Shizuoka 425-0013, Japan; [email protected] (H.S.); [email protected] (H.H.); [email protected] (A.O.-K.); [email protected] (Y.T.) 2 Department of Pharmacy, The University of Tokyo Hospital, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; [email protected] (T.T.); [email protected] (H.S.) * Correspondence: [email protected] These authors contributed equally to this work. y Received: 2 July 2020; Accepted: 12 August 2020; Published: 14 August 2020 Abstract: Axillary osmidrosis (AO) is a common chronic skin condition characterized by unpleasant body odors emanating from the armpits, and its aetiology is not fully understood. AO can seriously impair the psychosocial well-being of the affected individuals; however, no causal therapy has been established for it other than surgical treatment. Recent studies have revealed that human ATP-binding cassette transporter C11 (ABCC11) is an AO risk factor when it is expressed in the axillary apocrine glands—the sources of the offensive odors. Hence, identifying safe ways to inhibit ABCC11 may offer a breakthrough in treating AO. We herein screened for ABCC11-inhibitory activities in 34 natural products derived from plants cultivated for human consumption using an in vitro assay system to measure the ABCC11-mediated transport of radiolabeled dehydroepiandrosterone sulfate (DHEA-S—an ABCC11 substrate). -

Kaempferol Exhibits Progestogenic Effects in Ovariectomized Rats May Fern Toh, Emma Mendonca, Sharon L
s & H oid orm er o t n S f a l o S l c a Journal of i n e r n u c o e J Toh et al., J Steroids Horm Sci 2014, 5:3 ISSN: 2157-7536 Steroids & Hormonal Science DOI: 10.4172/2157-7536.1000136 Research Article Open Access Kaempferol Exhibits Progestogenic Effects in Ovariectomized Rats May Fern Toh, Emma Mendonca, Sharon L. Eddie, Michael P. Endsley, Daniel D. Lantvit, Pavel A. Petukhov, and Joanna E. Burdette* Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, Chicago, IL 60607, USA *Corresponding author: Joanna E. Burdette, 900 S. Ashland Street (M/C 870) Chicago, IL 60607, USA, Tel: 312-996-6153; Fax: 312-996-7107; E-mail: [email protected] Received date: April 15, 2014, Accepted date: June 26, 2014, Published date: July 02, 2014 Copyright: © 2014 Toh MF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Objective: Progesterone (P4) plays a central role in women's health. Synthetic progestins are used clinically in hormone replacement therapy (HRT), oral contraceptives, and for the treatment of endometriosis and infertility. Unfortunately, synthetic progestins are associated with side effects, including cardiovascular disease and breast cancer. Botanical dietary supplements are widely consumed for the alleviation of a variety of gynecological issues, but very few studies have characterized natural compounds in terms of their ability to bind to and activate progesterone receptors (PR).