Bone Microstructure and Changes in Tissue Mineralization Throughout Adulthood
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PART 1 of Volume 13:6 June 2013
LANGUAGE IN INDIA Strength for Today and Bright Hope for Tomorrow Volume 13:6 June 2013 ISSN 1930-2940 Managing Editor: M. S. Thirumalai, Ph.D. Editors: B. Mallikarjun, Ph.D. Sam Mohanlal, Ph.D. B. A. Sharada, Ph.D. A. R. Fatihi, Ph.D. Lakhan Gusain, Ph.D. Jennifer Marie Bayer, Ph.D. S. M. Ravichandran, Ph.D. G. Baskaran, Ph.D. L. Ramamoorthy, Ph.D. Assistant Managing Editor: Swarna Thirumalai, M.A. Contents Drama in Indian Writing in English - Tradition and Modernity ... 1-101 Dr. (Mrs.) N. Velmani Reflection of the Struggle for a Just Society in Selected Poems of Niyi Osundare and Mildred Kiconco Barya ... Febisola Olowolayemo Bright, M.A. 102-119 Identity Crisis in Jhumpa Lahiri’s The Namesake ... Anita Sharma, M.Phil., NET, Ph.D. Research Scholar 120-125 A Textual Study of Context of Personal Pronouns and Adverbs in Samuel Taylor Coleridge’s “The Rime of the Ancient Mariner” ... Fadi Butrus K Habash, M.A. 126-146 Crude Oil Price Behavior and Its Impact on Macroeconomic Variable: A Case of Inflation ... M. Anandan, S. Ramaswamy and S. Sridhar 147-161 Using Exact Formant Structure of Persian Vowels as a Cue for Forensic Speaker Recognition ... Mojtaba Namvar Fargi, Shahla Sharifi, Mohammad Reza Pahlavan-Nezhad, Azam Estaji, and Mehi Meshkat Aldini Ferdowsi University of Mashhad 162-181 Language in India www.languageinindia.com ISSN 1930-2940 13:6 June 2013 Contents List i Simplification of CC Sequence of Loan Words in Sylheti Bangla ... Arpita Goswami, Ph.D. Research Scholar 182-191 Impact of Class on Life A Marxist Study of Thomas Hardy’s Novel Tess of the D’Urbervilles .. -

Probable Deletions (PDF
LIST OF PROBABLE ENTRIES IDENTIFIED TO BE DELETED FROM ELECTORAL ROLL DISTRICT NO & NAME :- 14 THIRUVANANTHAPURAM LAC NO & NAME :- 139 KOVALAM STATUS - S- SHIFT, E-EXPIRED , R- REPLICATION, M-MISSING, Q-DISQUALIFIED. LIST OF PROBABLE ENTRIES IDENTIFIED TO BE DELETED FROM ELECTORAL ROLL DISTRICT NO & NAME :- 14 THIRUVANANTHAPURAM LAC NO & NAME :- 139 KOVALAM PS NO & NAME :- 1 L M S Lower Primary School, Muttakkadu SL.NO NAME OF ELECTOR RLN RELATION NAME SEX AGE IDCARD_NO STATUS 301 Anil George F Vikdar George M 48 GPL1733997 R 364 Lekshmi Y H Sumesh F 23 SHB0767129 R 451 Binu B S F Balachandran M 26 SHB0121749 R 491 Sherin Star M Vasantha Star M 32 GPL1891860 R 631 Satheesh F Pushparaj M 28 GPL1945930 S 736 Apsara A S F Ahoskan F 24 SHB0132829 R 816 Vasanthi V H Sathyadas F 52 CPL1330406 R 833 Leela J H Natesan F 65 SHB0758789 R 1001 Ajayakumar F Sivarajan M 44 KL/20/138/378535 R 1167 Noble T F Thomas M 24 SHB0853119 S STATUS - S- SHIFT, E-EXPIRED , R- REPLICATION, M-MISSING, Q-DISQUALIFIED. LIST OF PROBABLE ENTRIES IDENTIFIED TO BE DELETED FROM ELECTORAL ROLL DISTRICT NO & NAME :- 14 THIRUVANANTHAPURAM LAC NO & NAME :- 139 KOVALAM PS NO & NAME :- 2 S N Upper Primary School, Kattachalkuzhi SL.NO NAME OF ELECTOR RLN RELATION NAME SEX AGE IDCARD_NO STATUS 14 Lalu F Raimons M 32 GPL1911585 S 35 Sunil F Surendran M 33 GPL1726900 R 36 Sivapriya F Sivarajan F 28 GPL2017838 R 64 Sheejakumari H Anil Kumar F 38 GPL1944487 S 66 Krishnamma H Johnson F 72 SHB0017756 S 79 Soumya F Udayababu F 30 GPL1845957 R 92 Chellamma H Kuttan F 76 KL/20/138/321199 -

Current Affairs Capsule for SBI/IBPS/RRB PO Mains Exam 2021 – Part 2
Current Affairs Capsule for SBI/IBPS/RRB PO Mains Exam 2021 – Part 2 Important Awards and Honours Winner Prize Awarded By/Theme/Purpose Hyderabad International CII - GBC 'National Energy Carbon Neutral Airport having Level Airport Leader' and 'Excellent Energy 3 + "Neutrality" Accreditation from Efficient Unit' award Airports Council International Roohi Sultana National Teachers Award ‘Play way method’ to teach her 2020 students Air Force Sports Control Rashtriya Khel Protsahan Air Marshal MSG Menon received Board Puruskar 2020 the award NTPC Vallur from Tamil Nadu AIMA Chanakya (Business Simulation Game)National Management Games(NMG)2020 IIT Madras-incubated Agnikul TiE50 award Cosmos Manmohan Singh Indira Gandhi Peace Prize On British broadcaster David Attenborough Chaitanya Tamhane’s The Best Screenplay award at Earlier, it was honoured with the Disciple Venice International Film International Critics’ Prize awarded Festival by FIPRESCI. Chloe Zhao’s Nomadland Golden Lion award at Venice International Film Festival Aditya Puri (MD, HDFC Bank) Lifetime Achievement Award Euromoney Awards of Excellence 2020. Margaret Atwood (Canadian Dayton Literary Peace Prize’s writer) lifetime achievement award 2020 Click Here for High Quality Mock Test Series for IBPS RRB PO Mains 2020 Click Here for High Quality Mock Test Series for IBPS RRB Clerk Mains 2020 Follow us: Telegram , Facebook , Twitter , Instagram 1 Current Affairs Capsule for SBI/IBPS/RRB PO Mains Exam 2021 – Part 2 Rome's Fiumicino Airport First airport in the world to Skytrax (Leonardo -

Para Medical Certficate/Diploma Courses 2019 - 2020 Session Provisional Merit List for Eligible Non Service Candidates
PARA MEDICAL CERTFICATE/DIPLOMA COURSES 2019 - 2020 SESSION PROVISIONAL MERIT LIST FOR ELIGIBLE NON SERVICE CANDIDATES TOTAL COMMUNITY RANK ARNO COMMUNITY NAME MARKS RANK 1 374 NITHYA R BC 95.38 1 2 2038 PRAKASH R BC 92.00 2 3 82 RAJESWARI R BC 90.25 3 4 3702 ANNIE PUSHPA JEFFI A BC 90.13 4 5 3225 MUKILAN M BC 90.00 5 6 1798 MANIKANDAN M MBC/DNC 89.25 1 7 3415 MAHARAJAN P MBC/DNC 88.88 2 8 2433 SUMAIYA BEEVI A H BCM 88.75 1 9 2854 SEETHALAKSHMI M BC 88.00 6 10 3667 PAVITHRA K MBC/DNC 87.25 3 11 1160 NITHIYA R BC 87.13 7 12 3433 LENIJA J BC 87.13 8 13 3337 HARISH KUMAR O BC 87.00 9 14 4553 SIVAKUMAR M SCA 86.50 1 15 1738 AFROSE H BCM 86.38 2 16 1979 SINDHUJA P BC 85.75 10 17 3411 GOKULNATHAN S SC 85.50 1 18 1337 MANIGANDAN G MBC/DNC 85.25 4 19 1186 PRAKASH N SC 85.25 2 20 1411 SAPTHAGIRI S MBC/DNC 84.75 5 21 876 SARANYA R MBC/DNC 84.50 6 22 3675 KESAVAN V SCA 84.38 2 23 3664 SUREKA S BC 84.25 11 24 3839 ARUNKUMAR A SC 84.13 3 25 103 NOORULHITHAYA.M BCM 84.00 3 26 2468 PRICELA C BC 84.00 12 27 3887 PANDISELVI A BC 83.00 13 28 641 PRAVEEN K SC 83.00 4 29 906 DIVYA S MBC/DNC 83.00 7 30 585 NELSON D BC 82.75 14 Page 1 of 147 PARA MEDICAL CERTFICATE/DIPLOMA COURSES 2019 - 2020 SESSION PROVISIONAL MERIT LIST FOR ELIGIBLE NON SERVICE CANDIDATES TOTAL COMMUNITY RANK ARNO COMMUNITY NAME MARKS RANK 31 2958 UDAYAKUMAR D MBC/DNC 82.63 8 32 2573 MUNEESWARI S SC 82.50 5 33 1125 GOMATHI M MBC/DNC 82.50 9 34 2137 AASHIMA BEGAM S BCM 82.50 4 35 2984 KARTHEESWARI A BC 82.50 15 36 2711 TAMILVANAN K SC 82.38 6 37 3579 ABIMANI K MBC/DNC 82.00 10 38 4230 RAMAKRISHNAN -

Varsha Adalja Tr. Satyanarayan Swami Pp.280, Edition: 2019 ISBN
HINDI NOVEL Aadikatha(Katha Bharti Series) Rajkamal Chaudhuri Abhiyatri(Assameese novel - A.W) Tr. by Pratibha NirupamaBargohain, Pp. 66, First Edition : 2010 Tr. Dinkar Kumar ISBN 978-81-260-2988-4 Rs. 30 Pp. 124, Edition : 2012 ISBN 978-81-260-2992-1 Rs. 50 Ab Na BasoIh Gaon (Punjabi) Writer & Tr.Kartarsingh Duggal Ab Mujhe Sone Do (A/w Malayalam) Pp. 420, Edition : 1996 P. K. Balkrishnan ISBN: 81-260-0123-2 Rs.200 Tr. by G. Gopinathan Aabhas Pp.180, Rs.140 Edition : 2016 (Award-winning Gujarati Novel ‘Ansar’) ISBN: 978-81-260-5071-0, Varsha Adalja Tr. Satyanarayan Swami Alp jivi(A/w Telugu) Pp.280, Edition: 2019 Rachkond Vishwanath Shastri ISBN: 978-93-89195-00-2 Rs.300 Tr.Balshauri Reddy Pp 138 Adamkhor(Punjabi) Edition: 1983, Reprint: 2015 Nanak Singh Rs.100 Tr. Krishan Kumar Joshi Pp. 344, Edition : 2010 Amrit Santan(A/W Odia) ISBN: 81-7201-0932-2 Gopinath Mohanti (out of stock) Tr. YugjeetNavalpuri Pp. 820, Edition : 2007 Ashirvad ka Rang ISBN: 81-260-2153-5 Rs.250 (Assameese novel - A.W) Arun Sharma, Tr. Neeta Banerjee Pp. 272, Edition : 2012 Angliyat(A/W Gujrati) ISBN 978-81-260-2997-6 Rs. 140 by Josef Mekwan Tr. Madan Mohan Sharma Aagantuk(Gujarati novel - A.W) Pp. 184, Edition : 2005, 2017 Dhiruben Patel, ISBN: 81-260-1903-4 Rs.150 Tr. Kamlesh Singh Anubhav (Bengali - A.W.) Ankh kikirkari DibyenduPalit (Bengali Novel Chokher Bali) Tr. by Sushil Gupta Rabindranath Tagorc Pp. 124, Edition : 2017 Tr. Hans Kumar Tiwari ISBN 978-81-260-1030-1 Rs. -

NAAC Re-Accreditation Self Study Report
UNIVERSITY COLLEGE THIRUVANANTHAPURAM - 695 034 SELF STUDY REPORT SUBMITTED FOR REACCREDITATION TO NATIONAL ASSESSMENT AND ACCREDITATION COUNCIL BANGALORE - 560 072 2 NAAC Re-Accreditation Committee University College, Thiruvananthapuram Dr. B. S. Mohanachandran (Principal) Patron (Ex-officio) Dr. R. Anilkumar (Dept. of Geography) General Convenor Dr. K. P. Jaikiran (Dept. of Geology) Co-ordinator, IQAC Members Dr.S. Unnikrishnan Nair - (Vice-Principal) Sri. G. Rajeev - (Dept. of Chemistry) Sri. K. Gopalakrishnan - (Dept. of English) Dr. Thomas Kuruvilla - (Dept. of English) Dr. Francis Sunny - (Dept. of Zoology) Dr. Philip Samuel - (Dept. of Statistics) Sri. M.B. Salim - (Dept. of Geography) Sri. P. Surendran - (Dept. of Physical Education) 3 Contents Page No. PREFACE Part I - INSTITUTIONAL DATA 01 - 44 Profile of the Institution Criterion wise input Profiles of the departments Part II - EVALUATIVE REPORT 45 – 400 Stand out facts Executive summary Criterion wise evaluative report Evaluative report of Departments Declaration by the Principal 4 PREFACE University College, Thiruvananthapuram (estd.1866) occupies a position of eminence among the colleges in the state of Kerala and that of a hallowed alma mater among the millions of students, including luminaries like the late Dr K R Narayanan, the former President of India and Dr. G Madhavan Nair, former Director, Indian Space Research Organisation. The college, situated in the heart of Trivandrum, the capital city of Kerala is unique in more than one respect: more than sixty per cent of its teachers are research degree holders; the college has fourteen research departments offering M.Phil. and PhD; and its student strength of 3200* includes enrolment from all social classes. -
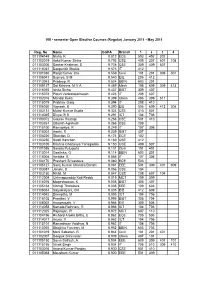
8 Sem Key List
VIII - semester Open Elective Courses (Regular) January 2011 - May 2011 Reg. No Name CGPA Branch 1 2 3 4 011104149 Nisha, K 9.812 ECE 503 402 302 011103019 Ankit Kumar Sinha 9.793 CSE 409 207 607 108 011103206 Sankar Krishnan, S 9.728 CSE 309 409 507 011115027 Deepankit Shukla 9.573 IT 412 011101030 Ranjit Kumar Jha 9.559 Civil 101 201 309 501 011106041 Supriya, S M 9.540 EIE 206 412 011113043 Pradeep, R 9.524 BBIN 603 201 011109127 Sai Krishna, M V A 9.469 Mech 108 609 309 413 011116055 Ishita Sinha 9.437 BIBT 309 407 011115078 Piduri Venkatasatheesh 9.423 IT 208 607 011102016 Mandal Keka 9.399 Chem 406 309 511 011115079 Prabhav Garg 9.394 IT 208 410 011106050 Vignesh, K 9.390 EIE 506 609 412 308 011103131 Mukul Kumar Gupta 9.324 CSE 410 501 011114045 Divya, B S 9.291 ICT 104 706 011103072 Gaurav Rastogi 9.268 CSE 501 410 011103261 Utkarsh Agnihotri 9.268 CSE 209 011115100 Ramapriya, R 9.249 IT 107 306 011116001 Aarthi, R 9.239 BIBT 407 011104220 Shantan, D 9.174 ECE 102 011103250 Swati Kanchan 9.169 CSE 412 011103105 Krishna Chaitanya Yarlagadda 9.150 CSE 409 507 011101036 Sarada Pulugurta 9.131 Civil 101 401 011113014 Geethika, G 9.114 BBIN 603 201 011115008 Ambika, B 9.085 IT 107 306 011104170 Prashant Srivastava 9.080 ECE 503 011105127 Saroj Kumar Chandra Banshi 9.061 EEE 501 309 401 609 011103087 Janani, S 9.056 CSE 104 011103150 Nikhil, M 9.047 CSE 208 607 104 011112009 Chinnapureddy Koti Reddy 9.019 MCT 109 309 011116076 Mageshwaran, K 9.005 BIBT 305 407 011105168 Vibhuji Thotakura 9.005 EEE 109 603 011106034 Satyakalyani, CH -

INDIAN OVERSEAS BANK Page 1 of 26 Direct Recruitment Register As on 31.12.2012
INDIAN OVERSEAS BANK Human Resources Development Department Page 1 of 26 No 763, Anna Salai , Chennai-600002 Direct Recruitment Register as on 31.12.2012 - Post :SWEEPER - ONE THIRD State : ANDHRA PRADESH Cycle No SL No UR or Reserved for Emp No Emp Name Recruitment Dt Caste_Category Reserved 1 1 UR 21755 INDRANI A 11.10.1999 OBC OBC-1 1 2 UR 22077 APPAYAMMA S 15.04.2004 OBC OBC-2 1 3 UR 22160 SHAIK ALIMA 13.08.2004 UR UR 1 4 OBC-1 22140 NARASAMMA K 15.09.2004 OBC OBC-3 1 5 UR 22218 MADDIKONDA BHAGYAVAT 02.05.2006 ST ST-1 1 6 UR 22257 VARALAKSHMI R 15.06.2006 OBC OBC-4 1 7 SC-1 22589 SAVITRAMMA S 24.01.2008 UR UR 1 8 OBC-2 22738 GORLE NARASIMHA MURT 24.01.2008 OBC OBC-5 1 9 UR 22745 CHUKKA SRINIVASA RAO 01.08.2008 OBC OBC-6 1 10 UR 22722 SANGEETHA RAO B 06.08.2008 UR UR 1 11 UR 22861 CHALLA RAMA RAO 19.05.2009 ST ST-2 1 12 OBC-3 22892 KONA LAKSHMI 28.08.2009 SC SC-1 1 13 SC-2 22900 CHIRANJI PADAL B 19.10.2009 ST ST-3 SUMMARY OF ROSTER Post : SWEEPER - ONE THIRD TOTAL 13 SC ST OBC UR 1 Percentage Of Reservation 16 7 27 - 2 No. Of Posts Reserved 2 0 3 8 3 No. Of Posts Utilised 1 3 6 3 Shortfall(-)/Excess(+) of 4 -1 3 3 - Reservation INDIAN OVERSEAS BANK Human Resources Development Department Page 2 of 26 No 763, Anna Salai , Chennai-600002 Direct Recruitment Register as on 31.12.2012 - Post :SWEEPER - ONE THIRD State : ASSAM Cycle No SL No UR or Reserved for Emp No Emp Name Recruitment Dt Caste_Category Reserved 1 1 UR 21867 CHANDRAMA BASFORE 09.12.1999 SC SC-1 1 2 UR 22081 TARUL RABIDASS 15.04.2004 SC UR SUMMARY OF ROSTER Post : SWEEPER - ONE THIRD TOTAL 2 SC ST OBC UR 1 Percentage Of Reservation 7 12 27 - 2 No. -

Alphabetical List of Recommendations Received for Padma Awards - 2014
Alphabetical List of recommendations received for Padma Awards - 2014 Sl. No. Name Recommending Authority 1. Shri Manoj Tibrewal Aakash Shri Sriprakash Jaiswal, Minister of Coal, Govt. of India. 2. Dr. (Smt.) Durga Pathak Aarti 1.Dr. Raman Singh, Chief Minister, Govt. of Chhattisgarh. 2.Shri Madhusudan Yadav, MP, Lok Sabha. 3.Shri Motilal Vora, MP, Rajya Sabha. 4.Shri Nand Kumar Saay, MP, Rajya Sabha. 5.Shri Nirmal Kumar Richhariya, Raipur, Chhattisgarh. 6.Shri N.K. Richarya, Chhattisgarh. 3. Dr. Naheed Abidi Dr. Karan Singh, MP, Rajya Sabha & Padma Vibhushan awardee. 4. Dr. Thomas Abraham Shri Inder Singh, Chairman, Global Organization of People Indian Origin, USA. 5. Dr. Yash Pal Abrol Prof. M.S. Swaminathan, Padma Vibhushan awardee. 6. Shri S.K. Acharigi Self 7. Dr. Subrat Kumar Acharya Padma Award Committee. 8. Shri Achintya Kumar Acharya Self 9. Dr. Hariram Acharya Government of Rajasthan. 10. Guru Shashadhar Acharya Ministry of Culture, Govt. of India. 11. Shri Somnath Adhikary Self 12. Dr. Sunkara Venkata Adinarayana Rao Shri Ganta Srinivasa Rao, Minister for Infrastructure & Investments, Ports, Airporst & Natural Gas, Govt. of Andhra Pradesh. 13. Prof. S.H. Advani Dr. S.K. Rana, Consultant Cardiologist & Physician, Kolkata. 14. Shri Vikas Agarwal Self 15. Prof. Amar Agarwal Shri M. Anandan, MP, Lok Sabha. 16. Shri Apoorv Agarwal 1.Shri Praveen Singh Aron, MP, Lok Sabha. 2.Dr. Arun Kumar Saxena, MLA, Uttar Pradesh. 17. Shri Uttam Prakash Agarwal Dr. Deepak K. Tempe, Dean, Maulana Azad Medical College. 18. Dr. Shekhar Agarwal 1.Dr. Ashok Kumar Walia, Minister of Health & Family Welfare, Higher Education & TTE, Skill Mission/Labour, Irrigation & Floods Control, Govt. -

Tnpsc Current Affairs - English March-2021 the Way to Your Destiny | Since 2014
TNPSC CURRENT AFFAIRS - ENGLISH MARCH-2021 THE WAY TO YOUR DESTINY | SINCE 2014 S.NO INDEX PAGE NO 1. TAMILNADU NEWS 2 2. SPECIAL NEWS 9 3. IMPORTANT EVENTS 18 4. PERSON IN NEWS 29 5. ECONOMIC AFFAIRS 37 6. SCIENCE & TECHNOLOGY 42 7. NATIONAL NEWS 45 8. INTERNATIONAL NEWS 56 9. OTHER STATE NEWS 66 10. SPORTS NEWS 75 11. IMPORTANT DAYS 82 TNPSC CURRENT AFFAIRS – ENGLISH MARCH-2021 Page | 1 THE WAY TO YOUR DESTINY | SINCE 2014 1. TAMIL NADU NEWS Activities which held up in Tamil Nadu during march 2021 VOC Port signs 45 MoUs for Rs. 27,000 crores investment The flagship initiative of the Ministry of Ports, Shipping & Waterways, Government of India, and Maritime India Summit-2021 is being organized on a virtual platform from 2nd to 4th March 2021, showcasing the global investment opportunities in the Indian Maritime Sector. Chennai, Coimbatore 'most liveable' cities in govt's Ease of Living Index The Ministry of Housing and Urban Affairs on March 4, 2021, released the Ease of Living Index 2020, which provided a ranking of the cities with a population of more than a million and a population of less than a million. In the million-plus cities, Bengaluru, Pune, and Ahmedabad have been ranked as the most livable cities in the country, while in less than a million categories, Shimla followed by Bhubaneshwar, Silvassa, Kakinada has been ranked as the most livable city. A total of 111 cities of India were judged in the Ease of Living Index 2020. The Ministry also released the ‘Municipal Performance Index’ under which the New Delhi Municipal Council topped in less than million population category and Indore topped in the million-plus population category. -

Padma Awards - One of the Highest Civilian Awards of the Country, Are Conferred in Three Categories, Namely, Padma Vibhushan, Padma Bhushan and Padma Shri
Padma Awards - one of the highest civilian Awards of the country, are conferred in three categories, namely, Padma Vibhushan, Padma Bhushan and Padma Shri. The Awards are given in various disciplines/ fields of activities, viz.- art, social work, public affairs, science and engineering, trade and industry, medicine, literature and education, sports, civil service, etc. ‘Padma Vibhushan’ is awarded for exceptional and distinguished service; ‘Padma Bhushan’ for distinguished service of high order and ‘Padma Shri’ for distinguished service in any field. The awards are announced on the occasion of Republic Day every year. 2. These awards are conferred by the President of India at ceremonial functions which are held at Rashtrapati Bhawan usually around March/ April every year. This year the President of India has approved conferment of 127 Padma Awards including one duo case (counted as one) as per the list below. The list comprises of 2 Padma Vibhushan, 24 Padma Bhushan and 101 Padma Shri Awardees. 27 of the Awardees are women and the list also includes 10 persons from the category of foreigners, NRIs, PIOs and Posthumous Awardees. Padma Vibhushan Sl No. Name Discipline State/ Domicile 1. Dr. Raghunath A. Mashelkar Science and Engineering Maharashtra 2. Shri B.K.S. Iyengar Others-Yoga Maharashtra Padma Bhushan Sl No. Name Discipline State/ Domicile 1. Prof. Gulam Mohammed Sheikh Art - Painting Gujarat 2. Begum Parveen Sultana Art - Classical Singing Maharashtra 3. Shri T.H. Vinayakram Art - Ghatam Artist Tamil Nadu 4. Shri Kamala Haasan Art-Cinema Tamil Nadu 5. Justice Dalveer Bhandari Public Affairs Delhi 6. Prof. Padmanabhan Balaram Science and Engineering Karnataka 7. -

INDIAN OVERSEAS BANK Page 1 of 87 Direct Recruitment Register As
INDIAN OVERSEAS BANK Human Resources Development Department Page 1 of 87 No 763, Anna Salai , Chennai-600002 Direct Recruitment Register as on 31.12.2012 - Post :CLERICAL-PROMOTEE State : ANDAMAN & NICOBAR ISL. Cycle No SL No UR or Reserved for Emp No Emp Name Recruitment Dt Caste_Category Reserved 1 1 UR 29888 SELVARAJAN V 03.03.2010 UR UR SUMMARY OF ROSTER Post : CLERICAL-PROMOTEE TOTAL 1 SC ST OBC UR 1 Percentage Of Reservation 15 7.5 0 - 2 No. Of Posts Reserved 0 0 0 1 3 No. Of Posts Utilised 0 0 0 1 Shortfall(-)/Excess(+) of 4 0 0 0 - Reservation INDIAN OVERSEAS BANK Human Resources Development Department Page 2 of 87 No 763, Anna Salai , Chennai-600002 Direct Recruitment Register as on 31.12.2012 - Post :CLERICAL-PROMOTEE State : ANDHRA PRADESH Cycle No SL No UR or Reserved for Emp No Emp Name Recruitment Dt Caste_Category Reserved 1 1 UR 5475 PRASADA RAO B V 05.03.1973 SC SC-1 1 2 UR 5705 CHANDRIAH B 16.08.1979 UR UR 1 3 UR 5872 BHASKARUDU T V 16.08.1979 UR UR 1 4 UR 5899 SATHYANARAYANA MURTH 17.08.1979 UR UR 1 5 UR 6136 APPA RAO G 15.09.1980 SC SC-2 1 6 UR 5883 TATA RATHNAM M 21.12.1981 SC SC-3 1 7 SC-1 6569 ANANTHARAJ S 21.12.1981 SC SC-4 1 8 UR 6705 VENKATESWARA RAO D 21.12.1981 UR UR 1 9 UR 6925 MANICKYALU RAO K V M 21.12.1981 UR UR 1 10 UR 6945 ACHARYULU D B B 21.12.1981 UR UR 1 11 UR 6771 SATYANARAYANA V 28.12.1982 UR UR 1 12 UR 6834 SATYANANDAM K 28.12.1982 SC SC-5 1 13 UR 25107 KAMESWARA RAO T V S 28.12.1982 UR UR 1 14 ST-1 6141 PRASAD RAJU CH 31.12.1983 UR UR 1 15 SC-2 6242 MUNI REDDY D 31.12.1983 UR UR 1 16 UR 6564 RAMBABU