Thermal Decomposition Behavior and Kinetic Study of Jamadoba Coal and Its Density Separated Macerals: a Non-Isothermal Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Government of India
Contents: Sl. No Topic Page No 1 General characteristics of the District 1 1.1 Location & geographical area 1 1.2 Topography 1 1.3 Availability of Minerals 2 1.4 Forest 2 1.5 Administrative setup 2 2 District at a Glance 3-5 2.1 Existing status of Industrial Area in the District 6 3 Industrial Scenario of …. 6 3.1 Industry at a glance 6 3.2 Year wise trend of Units registered 7 3.3 Details of existing Micro & small enterprises & Artisan Units in the 8 District 3.4 Large Scale Industries/ Public Sector Undertakings 9 3.5 Major Exportable Items 9 3.6 Growth Trend 10 3.7 Vendarization /Ancillarisation of the Industry 10 3.8 Medium Scale Enterprises 11 3.8.1 List of the units in Dhanbad & near by Area 11 3.8.2 Major Exportable Item 11 3.9 Service Enterprises 11 3.9.1 Coaching Industry --------- 3.9.2 Potential Areas for Service Industry 11 3.10 Potentials for New MSMEs 12-15 4 Existing clusters of Micro & Small Enterprise 15 4.1 Details of Major Clusters 15 4.1.1 Manufacturing Sector 15 4.1.2 Service Sector 15 4.2 Details of identified cluster 15 4.2.1 Refractory Cluster: Present Status, 15 5 General issues raised by the industry association during the course of 16 meeting 6 Steps to set up MSMEs 17 Brief Industrial Profile of Dhanbad District 1. General Characteristics of the District: Dhanbad District, an administrative district of Jharkhand has it’s headquarter at Dhanbad. -
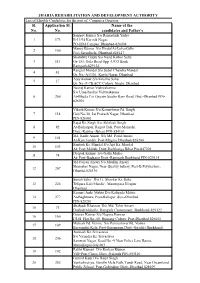
Sl. No. Application Sl. No. Name of the Candidates and Father's JHARIA
JHARIA REHABILITATION AND DEVELOPMENT AUTHORITY List of Eligible Candidates for the post of Computer Operator Sl. Application Sl. Name of the No. No. candidates and Father's Sanjeev Kumar S/o Ramashish Yadav 1 173 B-11/94 Karmik Nagar, PO-ISM Campus, Dhanbad-826004 Manoj Kumar S/o HiralalAt-Loharkulhi 2 100 Post-Saraidhela, Dhanbad-828127 Shambhu Gupta S/o Nand Kishor Sahu 3 113 Gr-153, Gola Road Opp.-UCO Bank Ramgarh-829122 Ranglal Mandal S/o Subal Chandra Mandal 4 41 Qr. No.-A1/301, Koyla Nagar, Dhanbad Ajay Kumar S/o Krishna Sahu 5 17 Qr. No.-F-7B ACC Colony, Sindri, Dhanbad Neeraj Kumar Vishwakarma S/o Umashankar Vishwakarma 6 260 At-Bhuda C/o Gayatri Studio Rani Road, Dist.-Dhanbad PIN- 826001 Vikash Kumar S/o Kameshwar Pd. Singh 7 114 Gali No-10, Jai Prakash Nagar, Dhanbad PIN-826001 Rajat Kr. Singh S/o Akhilesh Singh 8 92 At-Bishanpur, Rajput Tola, Post-Mansahi, Distt.-Katihar (Bihar) PIN-854103 Md. Kadir Ansari S/o Md. Farid Ansari 9 101 At-Kanchandih, Post-Mugma Dhanbad-828204 Santosh Kr. Mandal S/o Ajit Kr. Mandal 10 103 At+Post-Mahuli, Distt-Darbhanga Bihar Pin-847201 Deepak Kumar S/o Gallu Mahto 11 74 At+Post-Badgaon Distt.-Ramgarh Jharkhand PIN-829134 Md Faiyaz Ansari S/o Muslim Ansari Shamsher Nagar, Near Quality bakery, Post-B.Polytechnic, 12 267 Dhanbd-828130 Sonali Saha D/o Lt. Shankar Kr. Saha 13 224 Telipara Kali Mandir , Masterpara Hirapur Dhanbad Kumari Janki Mahto D/o Kalipada Mahto 14 277 At-Baghmara, Post-Baliapur, dist.-Dhanbad PIN-828201 Shahjadi Khatoon D/o Md. -

BHARAT COKING COAL LIMITED (A Subsidiary of Coal India Limited) OFFICE of the G.M., E.J
BHARAT COKING COAL LIMITED (A subsidiary of coal India limited) OFFICE OF THE G.M., E.J. AREA PO. BHOWRA (DHANBAD) Ref. no. – GM(EJA) ACE/NIT/10-11/462 Dated: - 31.01.2011 Notice inviting Tender Sealed tenders in two parts ( Part-I & Part –II) on overall percentage rate basis are invited from experienced and eligible contractors for the following works. Cost of Sl. Estimated Time of Name of work E.M tender No. amount Completion paper 1. Repair of overhead structure Rs 68,075.03 Rs 681.00 Rs. 100 1 month at KM 319/J 12-13 between Bhowra – Jamadoba SE Rly. Track under EJ Area AVAILABILITY OF TENDER DOCUMENTS From 11.02.2011 to 12.02.2011 Tender documents will be issued on all working days during working hours except on Sunday & holiday in the event of thr specified date for submission, opening of bids being declared a holiday the bids will be received opened on the appointed time on the next working day. The Tender document is also available on web site : http://bccl:cmpdi:co:in and can be downloaded. TIME & DATE IF RECEIPT/OPENING OF TENDER The Tender will be received on 15.02.2011 from 10.00AM to 1.00PM in Tender box at (1). CISF post near Koyla Bhawan gate, Koyla Nagar, BCCL, Dhanbad. (2). CISF’S office of EJ Area and will be opened on 18.02.2011 at 4.00 PM in the office of the ACE/EJA 1. ELIGIBILITY CRITERIA a). The intending Tenderer must have in its name as a prime contractor experience of having successfully completed similar works during last 7(seven) years ending last day of month previous to the one in which bid applications are invited should be either of the following :- (1). -

Jamadoba Coal Washery (Capacity: Expansion from 1 to 2 Mtpa Raw Coal Throughput) Tehsil: Jharia, Dist: Dhanbad, Jharkhand
HALF YEARLY COMPLIANCE REPORT (PERIOD: APRIL’19 – SEPTEMBER’19) JAMADOBA COAL WASHERY (CAPACITY: EXPANSION FROM 1 TO 2 MTPA RAW COAL THROUGHPUT) TEHSIL: JHARIA, DIST: DHANBAD, JHARKHAND TATA STEEL LIMITED, JHARIA DIVISION P.O.- JAMADOBA, DIST. - DHANBAD, STATE- JHARKHAND, PIN CODE – 828112. ENVIRONMENTAL CLEARANCE GRANTED VIDE LETTER NO. - J-11015/203/2011- IA.II(M) DATED- 03.03.2014 ISSUED BY GOVT. OF INDIA, MINISTRY OF ENVIRONMENT, FOREST AND CLIMATE CHANGE, NEW DELHI. Compliance status of Expansion of Jamadoba Coal washery (1 MTPA to2 MTPA in 7 ha Area) of M/s Tata Steel Ltd. Tehsil Jharia, dist. Dhanbad, Jharkhand vide MoEFCC Letter no J- 11015/203/2011-IA.II (M) dated March 3, 2014 S. No. Condition Compliance Status Specific Condition Adequate green belt shall be provided Greenery has been developed in many areas around coal handling and other areas. around the washery premises. Since the expansion job has been completed, remaining (i) area is also being developed since last monsoon. The report on greenbelt development with photographs is provided in Annexure-I. Action plan be developed and Action plan for cut-off of tailing ponds from implemented for abandoned tailing ponds tailing management system is in place. and for their restoration to the original Abandoned tailing ponds have been reclaimed by (ii) land use. backfilling. Technical reclamation have taken place in two large ponds. Biological reclamation is going on by planting native species in this monsoon season. Fly ash generated from the captive power This is not applicable as we have ceased the plant of the washery be utilized for house operation of our captive Jamadoba power plant construction, roads and such uses, strictly since April’15. -

Change Detection Analysis of Ground Water Quality And
CHANGE DETECTION ANALYSIS OF GROUND WATER QUALITY AND ITS MANAGEMENT IN THE DISTRICT DHANBAD, JHARKHAND, INDIA Shiv Kumar Gupta1, Kumar Nikhil2, Aditya Shrestkar3, Gaurav Gehlot4 1,3,4Students of B.Tech.(Final) in Environmental Science & Engineering, ISM, Dhanbad, Jharkhand, (India) 2Principal Scientist, CSIR-CIMFR, Barwa Road, Dhanbad, Jharkhand, (India) ABSTRACT Dhanbad, a major coal mining area of India for more than a century, is situated in the middle of the Damodar river basin. The thickness, size, extent and openness of the weathered zone and the interconnections of fractures govern the groundwater movement in the Dhanbad area of Jharkhand and the depth to ground water generally ranges from 2 to 10 m below ground level. Urbanisation and the unregulated growth of the population have altered the terrain and slope morphology of the area. As a consequence, changes have taken place in the surface drainage system which indirectly affects the hydrogeology while the water infiltration ratio has resulted in a lowering of the groundwater. Inadequate environmental protection measures in the coal mining and related industries as well as the presence of active and abandoned coal mines, waste dumps, coal washeries, coking coal plants, thermal power plants, refractories, steel, fertilizer and cement plants have resulted in significant water pollution. In this paper study had been conducted in all the nine block of district Dhanbad. Randomly five villages were selected from each block with well or handpump were selected according to the availability and water sampling, testing and analysis done for pH, EC, TDS, Salinity, Fe, Mn and Zn availability followed by its assessmentand management. -

Tata Steel Limited Jharia Division
TATA STEEL LIMITED JHARIA DIVISION Page | 1 TATA STEEL LIMITED JHARIA DIVISION HALF YEARLY COMPLIANCE REPORT OF DIGWADIH COLLIERY TATA STEEL LIMITED, JHARIA DIVISION P.O.- JEALGORA, DIST. - DHANBAD, STATE- JHARKHAND, PIN CODE – 828110. ENVIRONMENTAL CLEARANCE GRANTED VIDE LETTER NO. - J-11015/372/2010-IA.II(M) DATED- 30TH SEPTEMBER 2013 ISSUED BY GOVT. OF INDIA, MINISTRY OF ENVIRONMENT & FOREST, NEW DELHI. Page | 2 TATA STEEL LIMITED JHARIA DIVISION A. SPECIFIC CONDITIONS: (i) The maximum production from the mines shall not exceed beyond that for which environmental clearance has been granted. It will be strictly followed. (ii) The impact on the Damodar river due to mining activity which is at a distance of 2 km be studied through a reputed 3rd Party and submitted to the SPCB and the regional office of the MoEF for monitoring. The underground coal mines operated by Tata Steel Ltd are located at a distance of 2-3 kms from the Damodar River. Other coal mines of BCCL, SAIL are also in close proximity to the river basin. Hence, there is a cumulative impact on the river due to the mining activity carried out in the Jharia coalfield. We have undertaken a project to determine the impact of mining activity with the help of the reputed organization, Indian School of Mines, Dhanbad who is conducting the study and will suggest measures on minimizing the impact. The report will be submitted next year. (iii) Details of underground transportation of coal from mine to coal yard/ rail yard be submitted to the MoEF for record. Coal from the mine is sent to the captive Jamadoba coal washery for beneficiation purpose through a network of underground conveyor belts. -

For the Year Ended 31 March 2014
Report of the Comptroller and Auditor General of India on General, Social and Economic (Non-PSUs) Sectors for the year ended 31 March 2014 Government of Jharkhand Report No. 2 of the year 2015 TABLE OF CONTENTS Reference to Paragraph Page Preface v Overview vii CHAPTER – 1 INTRODUCTION Budget profile 1.1.1 1 Application of resources of the State Government 1.1.2 1 Persistent savings 1.1.3 2 Funds transferred directly to the State implementing agencies 1.1.4 2 Grants-in-aid from Government of India 1.1.5 3 Planning and conduct of audit 1.1.6 3 Lack of responsiveness of Government to Inspection Reports 1.1.7 3 Follow-up on Audit Reports 1.1.8 4 Government response to significant audit observations (draft 1.1.9 5 paragraphs/reviews) Status of placement of Separate Audit Reports of Autonomous Bodies 1.1.10 6 in the State Assembly CHAPTER – 2 PERFORMANCE AUDIT DRINKING WATER & SANITATION DEPARTMENT Total Sanitation Campaign/Nirmal Bharat Abhiyan 2.1 7 LABOUR, EMPLOYMENT & TRAINING DEPARTMENT AND SCIENCE AND TECHNOLOGY DEPARTMENT Establishment and Upgradation of Government Women ITIs and 2.2 25 Government Women Polytechnics in Jharkhand HUMAN RESOURCES DEPARTMENT (HIGHER EUUCATION) Functioning of State Universities in Jharkhand 2.3 38 FOREST AND ENVIRONMENT DEPARTMENT Compliance with Environmental Laws in Dhanbad district including 2.4 66 Dhanbad Agglomeration SOCIAL WELFARE, WOMEN & CHILD DEVELOPMENT DEPARTMENT AND PLANNING & DEVELOPMENT DEPARTMENT Implementation of Schemes for Welfare and Protection of Girls in 2.5 77 Jharkhand HOME DEPARTMENT Information Technology Audit on preparedness of Crime and 2.6 94 Criminal Tracking Network System HUMAN RESOURCE DEVELOPMENT DEPARTMENT AND HEALTH, MEDICAL EDUCATION & FAMILY WELFARE DEPARTMENT Tribal Sub Plan (Education and Health Sectors) 2.7 105 ENERGY DEPARTMENT Implementation of Solar Energy programmes in Jharkhand 2.8 116 Audit Report on General, Social and Economic (Non-PSUs) Sectors for the year ended 31 March 2014 CHAPTER – 3 COMPLIANCE AUDIT Non-Compliance with the Rules, Orders, etc. -

Risk Analysis on the Use of Damodar River Water for Drinking Purposes
International Journal of Current Engineering and Technology E-ISSN 2277 – 4106, P-ISSN 2347 - 5161 ® ©2014 INPRESSCO , All Rights Reserved Available at http://inpressco.com/category/ijcet Research Article Risk Analysis on the use of Damodar River Water for Drinking Purposes Anubha SinghȦ*, Bishnu DeoḂ and S.P.SinghĊ ȦDepartment of Applied Chemistry, BIT Patna Campus, Patna – 800 014, INDIA ḂPDIL, Sindri, Dhanbad – 828 122, Jharkhand, INDIA ĊDepartment Chemical Engg. BIT Sindri, Dhanbad – 828 123, Jharkhand, INDIA Accepted 20 February 2014, Available online 25 February 2014, Vol.4, No.1 (February 2014) Abstract The paper shows the incorporation of various influencing parameters in water quality. Damodar river is mainly polluted due to mine overburden, fly ash, oil, toxic metals and coal dust, that has been drastically controlled by strict implementation by the Central Pollution Control Board as well as State Pollution Control Board for the effective measures to be taken by the industries and municipal bodies. The study shows the effect of biological parameters like – Biological Oxygen Demand (BOD =Exposure Factor 24.61), Most Probable Number (MPN =Exposure Factor 32.42) and Total Kjeldahl Nitrogen (TKN = Exposure Factor 31) mainly responsible for the river pollution. Similarly, Surrogate Number Level of exposure Factor i.e., SNLF for BOD is 24.61, for MPN is 32.42 and for TKN is 31. The above parameters help in deciding mode of Hazard transmission, Condition, Nature of Risk, Consequences, Severity of Risk as per EF, Severity of Risk as per SNLF, Detectability and Risk Classification. The present study will be helpful for industrialists and environmentalists to analyze the hazard transmission and apply the risk control measures. -

Jitpur Colliery Location
JITPUR COLLIERY LOCATION: Jitpur Colliery of Collieries Division, M/s Steel Authority of India Ltd is located in the eastern part of the Jharia Coal field District Dhanbad, Jharkhand. The mine take area is around 1.62 Sq.Km. The area is covered by the Survey of India Toposheet No. 73 I/6 and is bounded by Latitude 23° 42’ 32” north to 23° 43’ 14” north and Longitude 86° 23’ 05” east to 86° 24’ 14” east. The property is bounded by existing Jamadoba 6 & 7 pits of TISCO to the North (Rise Side), Bhutgoria mine(closed) of BCCL to the North and North-West, Jamadoba colliery(TISCO) Pit 4&5 to the south (Dip Side) & West and Digwadih (TISCO) to the East. Jitpur Colliery is well connected by metal road with district head quarter Dhanbad. It is located at 15Km south of Dha 333nbad by road. Background: Jitpur Colliery has been in operation prior to nationalization and operation is still continuing. There are two mouzas under Jitpur Colliery viz. Noonudih Mouza (Area: 73.68 ha ) & Jitpur Mouza (Area: 87.31 ha). Both these Mouzas have been granted lease for 991 years w.e.f 22/05/1901 & 991 years w.e.f 28/05/1902 respectively. Jitpur Colliery Colliery consists of one underground mine only. Coal from Jitpur Colliery is being transported by ropeway to Chasnalla Washery for washing. Detailed exploration was conducted by drilling 21 exploratory bore holes in 1950s. Further, in 1959, five underground boreholes were drilled from XVI seam floor. The detailed geological investigations in the proposed mine has established the presence of 8 No. -

Action Plan for Clusters of Dhanbad
ACTION PLAN FOR CLUSTERS OF DHANBAD _________________________________________________ JHARKHAND STATE POLLUTION CONTROL BOARD T A BUILDING, HEC COMPLEX, RANCHI 834004 1 (1) 1.0 INTRODUCTION 1.1 The territorial area of Nirsa block , industrial area of Govindpur block, municipal area of Dhanbad Sadar block, municipal area of Jharia block and industrial area of Sindri are included in the cluster of Dhanbad 1.2 Location: Satellite imagery is as given below. SATELLITE IMAGERY OF INDUSTRIAL CLUSTER OF DHANBAD SATELLITE IMAGERY OF THE AREA OF NIRSA BLOCK 2 SATELLITE IMAGERY OF DHANBAD SADAR BLOCK SATELLITE IMAGERY OF INDUSTRIAL AREA OF SINDRI 3 SATELLITE IMAGERY OF JHARIA BLOCK SATELLITE IMAGERY OF THE AREA OF GOBINDPUR 1.3 Digital map with demarcation of geographical boundaries and impact zones is as given below. 4 1.4 CEPI Score: Air- 64.50, Water- 59.00, Land -65.50, Total- 78.63 1.5 Total populations and sensitive receptors Hospital – Patliputra Medical College & Hospital, Dhanbad;Central Hospital, Jagjivan Nagar; Jamadoba Hospital; Fertilizer Hospital, Sindri Educational Institution – BIT Sindri, ISM Dhanbad, Patliputra Medical College, Dhanbad, RS More College, Govindpur, Sindri College, RSP College, Jharia, PK Roy College, SSLNT Womens College, BS College, Govt. Polytechnic, Dhanbad, Mining Institute, Dhanbad, Govt. Polytechnic, Bhaga; Mining institute, Bhaga; and around ten other colleges. Besides, there are around 500 schools. Court - The court of District and Session Judge is there at Dhanbad. TABLE-1 Details of population, its density, major surface water bodies, tourist spots, schools and the health care units 5 Name of block areas Sadar Jharia Govindpur Nirsa T Area (Sq. Km) 128.82 90.77 334.44 416.85 a Population (2001) 564468 475341 201876 376843 b Densityl 4382 5224 604 904 Majore Surface water Damodar, Damodar, Damodar, Damodar, body Barakar Barakar Barakar Barakar Tourist1 Spot NA NA NA Panchet & Maithan Dam Primary & Middle 155 119 144 215 School Healthcare unit 93 44 4 22 1.6 SourceE -- Govt. -

Jamadoba Colliery (Raw Coal Production of 0.34 MTPA) Under Modernisation
REVISED APPLICATION FOR PRIOR ENVIRONMENTAL CLEARANCE FOR Jamadoba Colliery (Raw Coal Production of 0.34 MTPA) Under Modernisation At Jharia Coal Field, Dhanbad District, Jharkhand Submitted by: M/s. Tata Steel Limited, Jamadoba, Dhanbad District, Jharkhand June 2019 1 APPENDIX I FORM I (I) Basic Information Sr. No. Item Details 1 Name of the project/s Modernization of Underground coal mining project - Jamadoba Colliery in Jharia coal field, Dhanbad district, Jharkhand State 2 Sr. No. in the schedule 1(a) 3 Proposed Capacity of the mine: 0.34 MTPA (raw coal capacity/area/length/tonnage to production). There will not be any increase in be handled/ command area/lease production after modernization of the area number of wells to be drilled underground mine. Mine Lease Area: 927.17 ha 4 New/Expansion/Modernization Modernization 5 Existing Capacity / Area etc. 0.34 MTPA of raw coal / Mine Lease Area: 927.17 ha 6 Category of Project i.e. ‘A’ or ‘B’ ‘A’ 7 Does it attract the general No condition? If yes, please specify. 8 Does it attract the specific No condition? If yes, please specify. 9 Location Coordinates: Points Latitude Longitude 1 23° 41' 53.3" N 86° 22' 09.5" E 12 23° 43' 17.0" N 86° 22' 08.9" E 15 23° 43' 35.9" N 86° 22' 16.5" E 55 23° 42' 43.1" N 86° 23' 58.8" E 63 23° 41' 04.6" N 86° 24' 46.4" E 66 23° 41' 06.6" N 86° 24' 30.4" E 89 23° 41' 22.0" N 86° 23' 08.8" E 90 23° 41' 21.4" N 86° 22' 58.1" E Plot / Survey / Khasra No. -

MANISH Final
DISTRICT SUPERINTENDENT OF EDUCATION, PALAMU foHkkxh; ladYi la[;k 1145 fnukad 18-07-2019 ds vkyksd esa vizf'kf{kr f'k{kdksa dks mudh fu;qfDr frfFk ls ,oa yksd lsok vk;ksx }kjk fu;qDr f'k{kdksa dk e/s kk lwph ls xzsM&1 dh vkilh ojh;rk lwphA Social Sl# Name School Block Gender DOB DOJ in Service Grade 1 Designation Sl.No. In Merit List Address Remarks Category MOHAMMAD MADRASATUL ISLAM KUDAGA Unreserv 1 CHAINPUR (200210) Male 12/05/1962 25/09/1980 25/09/1980 INTER TRAINED PATRATU,SARJU,GARU, LATEHAR JAMALUDIN KALAN ed NEZMUDDIN AHAMAD MADRASATUL ISLAM KUDAGA Unreserv 2 CHAINPUR (200210) Male 03/02/1962 01/10/1980 01/10/1980 INTER TRAINED SHAHPUR, CHAINPUR, PALAMU ANSARI KALAN ed MADRASATUL ISLAM KUDAGA Unreserv 3 MD FASIHUDDIN CHAINPUR (200210) Male 04/04/1964 01/06/1982 01/06/1982 INTER TRAINED SHAHPUR, CHAINPUR, PALAMU KALAN ed PRAMOD KUMAR BISHRAMPUR Unreserv 4 UPG RAJKIYEKRIT MS TONA Male 01/01/1963 21/08/1982 21/08/1982 INTER TRAINED DUBEY (200209) ed Unreserv 5 AMAR NATH JHA KALI SANSKRIT MS KISHUNPUR PATAN (200212) Male 05/06/1964 29/08/1982 29/08/1982 INTER TRAINED ed DALTONGANJ Unreserv 6 AHMAD HUSSAIN MADARSA AHSANUL ULOOM Male 23/04/1964 01/04/1983 01/04/1983 INTER TRAINED kund mohallah near millat masjid (200201) ed MD REYAZUDDIN MADRASATUL ISLAM KUDAGA Unreserv 7 CHAINPUR (200210) Male 12/11/1963 02/01/1984 02/01/1984 INTER TRAINED SUNTHA, PANDEYPURA, PATAN, PALAMU ANSARI KALAN ed UPG RAJKIYEKRIT MS MANJHI Unreserv VILL- MAJHIGAWAN, P.