Assessing the Impact of Oil Types and Grades on Tocopherol and Tocotrienol Contents in Vegetable Oils with Chemometric Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hazelnut: a Valuable Resource
International Journal of Food Engineering Vol. 6, No. 2, December 2020 Hazelnut: A Valuable Resource Raquel P. F. Guiné and Paula M. Reis Correia CI&DETS/ESAV, Polytechnic Institute of Viseu, Department of Food Industry, Viseu, Portugal Email: [email protected], [email protected] Abstract—Hazel (Corylus avellana L.) nutshell is one of the remaining 90% is used for industrial purposes as shelled most consumed and most appreciated nut fruit all over the nuts [7], [8]. world. It is believed to have constituted a basic food in early Similarly, to other nuts, hazelnuts are included in the prehistory, in temperate zones of the globe, such as for dietary guidelines of several countries, due to their high example Europe. Presently the hazelnut production is nutritional content and bioavailability of those nutrients. mainly concentrated on the Black Sea coast of Turkey, but other countries are also important producers, like for Furthermore, the bioactive molecules present in hazelnuts example Portugal, situated on the western Europe, in the have been reported as having several benefits for the Iberian Peninsula. The objective of this work is to make a human health [9]–[11]. review about the worldwide importance of hazelnut, their usages, including gastronomic and industrial applications, II. CONSUMPTION & MAIN USAGES as well as some ways that allow adding value to this fruit, making it an even more valuable resource. The advantages From the Neolithic era, in the regions of Europe and include higher income for produces, lower environmental the Caucasus, hazelnut has been used for human impacts and valorisation of residues improving consumption. -
Nutrient Comparison Chart
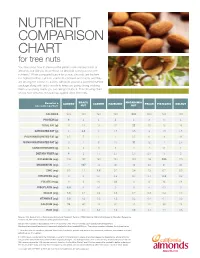
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

International Journal for Scientific Research & Development| Vol. 7, Issue 03, 2019 | ISSN (Online): 2321-0613
IJSRD - International Journal for Scientific Research & Development| Vol. 7, Issue 03, 2019 | ISSN (online): 2321-0613 Significance of Ethanol Blended Biodiesel Powered Diesel Engine Aditya Deshmukh1 Shrikrushna Dhakne2 Tohid Pathan3 Rahul Dimbar4 C. Srinidhi5 1,2,3,4UG Students 5Assistant Professor 1,2,3,4,5Department of Mechanical Engineering 1,2,3,4,5Suman Ramesh Tulsiani Tehnical Campus Faculty of Engineering Khamshet Pune, Maharashtra, India Abstract— In recent years, energy consumption is increasing engines. This chemical treatment is known as due to increase in the demand for the energy due to transesterification. Transesterification involves breaking of industrialization and increase in number of automobiles. This large triglycerides into a smaller Monoalkyl ester. The High leads to depletion of fossil fuels and hence researchers Viscosity of triglycerides can be reduced by the concentrate on alternative fuels which reduce the engine transesterification process in which triglyceride decompose emission and are environmentally friendly. This leads to into glycerol and glycerin, which is the biodiesel. The biodiesel produced from non-edible as an immediate biodiesel can be produced from a variety of edible and substitute to the fossil diesel. In this work, we used Kenaf oil nonedible oils, animal fats etc. The chosen oil will be for the production of the biodiesel as it has considerable subjected to transesterification in the existence of catalyst to potential for the production of the biodiesel. In this work, produce biodiesel. biodiesel was produced from the Kenaf oil using Indian consumption of petroleum fuels for the transesterification and we found that the properties of the period of 2008-09 was 30.50 million tons. -

Palm Oil and Rice Bran Oil: Current Status and Future Prospects
International Journal of Plant Physiology and Biochemistry Vol. 3(8), pp. 125-132, August 2011 Available online at http://www.academicjournals.org/ijppb ISSN-2141-2162 ©2011 Academic Journals Review Palm oil and rice bran oil: Current status and future prospects Kusum R., Bommayya H., Fayaz Pasha P. and Ramachandran H. D.* Department of Biochemistry, Dr. Ambedkar Veedhi Bangalore University, Bangalore - 560001, India. Accepted 17 June, 2011 The continued demand for edible oils by the ever increasing population makes it pertinent to explore new sources. In this direction, two new edible oils namely palm oil and rice bran oil have been subjected to nutritional and toxicological evaluations of their chemicals constituents. An attempt has been made in this article to assess the acceptability of the two oils based on the various investigations that have been carried out so far. Key words: Palm oil, rice bran oil, anti-oxidants, cholesterol fatty acids, phospholipids, tocopherols, oryzanol, cardiovascular diseases. INTRODUCTION Vegetable oils are the main source of dietary fat for Among the oils under consideration, palm oil and rice almost all sections of the Indian population and there is a bran oil offer great scope in India, as they are widely continued growing demand from both caterers and con- preferred by the vanaspathi industries and also by the sumers. Although the Indian population has a penchant Indian consumer. The oil palm gives higher yields in for a variety of deep fried products, there is also a greater comparison with other oil yielding species. Rice bran oil awareness of the problems such as atherosclerosis also offers high potential, as India has high rice caused by saturated fats. -

List of Legumes
Healthy Oils & SmokePoints When it comes to the cooking oil in your cupboard, there is a huge difference in healthfulness depending on how the oil is stored and how it will be used. First let’s get some definitions for commonly used terms on labels and discussions about oils. Term Definition Cold Pressed Extracted without using heat. Extracted using a screw-type machine that presses the oil out. Can be done Expeller Pressed slowly, with very little heat, or can be done quickly with lots of friction and high temperatures. The first cold pressing which contains the best tasting and most healthful oils. Must contain less than 1% acids. By definition, this is cold-pressed and first Extra Virgin pressed, so don’t need to see these terms on the label. Must say 100% extra virgin, or may be a blend. The first cold pressing, but can contain a little more acids than the extra-virgin Virgin (1-3 percent). Seeds that have been genetically manipulated to decrease the amount of High Oleic essential fatty acids so that they have a longer shelf life. Are left in their state after pressing – no filtering. These oils tend to be more Unrefined Oils flavorful and richer in nutrients, however they have a very low smoke point. Oils have their impurities filtered out, to increase stability and allow for higher Refined temperature cooking. The processing can use toxic solvents, caustic soda, bleaches and phosphoric acid. Smoke Point Stage at which a heated oil begins to smoke, just before it bursts into flames. HEALTHY OILS & SMOKE POINTS PAGE | 1 © 2021 Health-Naturally.org Term Definition Oil should smell and taste like the food it came from. -

Czech University of Life Sciences Prague
Czech University of Life Sciences Prague Faculty of Tropical AgriSciences Molecular Characterization of Plukenetia volubilis L. and Analysis of Seed Storage Protein Pattern and Protein Fractions Dissertation Thesis Department of Crop Sciences and Agroforestry Author: Ing. Martin Ocelák Supervisor: doc. Ing. Bohdan Lojka, Ph.D. Co-supervisors: Ing. Petra Hlásná Čepková, Ph.D. Ing. Iva Viehmannová, Ph.D. In Prague, September, 2016 Acknowledgment I would like to express my gratitude to my supervisor doc. Ing. Bohdan Lojka, Ph.D. and co-supervisors Ing. Petra Hlásná Čepková, Ph.D. and Ing. Iva Viehmannová, Ph.D. for their guidance, advices, help and also patience during the studies, laboratory works and mainly during the writings. My thanks also belong to IIAP represented by Ing. Danter Cachique Huansi and Lucas Garcia Chujutalli for their cooperation in samples collection, to Ing. Anna Prohasková for her guidance during analysis of proteins in Crop Research Institute in Prague - Ruzyně, to Ing. Eva Beoni, Ph.D. and Ing. Lenka Havlíčková, Ph.D. for their help in learning how to work in the laboratory; to Ing. Zdislava Dvořáková, Ph.D. for her help, teaching and encouragement and to Ing. Blanka Křivánková, Ph.D. for providing some useful materials. Also my family contributed with their support in all means. So great thanks belong to my parents Jan and Jaroslava Ocelákovi and my boyfriend Ioannis Nikolakis for their love and support in all possible means. This research was supported financially by an Internal Grant Agency of the University of Life Science Prague, CIGA (Project No. 20135004), by an Internal Grant Agency of the Faculty of Tropical AgriSciences - University of Life Science Prague, IGA (Project No. -

Cooking Oil Facts
Cooking Oil Facts As you enter a department store, you behold an array of cooking oils sporting all types of jargon on the packaging -- saturated fats, unsaturated fats, refined, filtered, ricebran oil, vanaspati, etc. Confused already? With so much variety and so many brands flooding the market today, buying the right cooking oil can prove a tough task. Different oils fill different needs - for health, taste and cooking. For good health, our bodies need a variety of healthy fats that are found naturally in different oils. When cooking, it's essential to know which oils are best for baking, sautéing and frying and which are healthiest used raw. Why have Oil (fats)? Contrary to popular belief, fat is actually a valuable part of one's diet, allowing people to absorb nutrients that require fat in order to metabolize in the body. Natural fats contain varying ratios of three types of fats: saturated, monounsaturated and polyunsaturated. • Saturated fats are hard at room temperature. They're stable, resist oxidation, and are found primarily in meat, dairy, palm and coconut oil. • Polyunsaturated fats are liquid at room temperature and the least stable. They oxidize easily and are found in seafood corn, safflower, soybean, and sunflower oils. • Monounsaturated fats are more stable than polyunsaturated fats. They're found in canola, nut and olive oils. It is recommended to limit saturated fats in the diet due to their association with cardiovascular disease. Also, you should try to rely more on monounsaturated than polyunsaturated fats. What are the varieties of Oil available in the market? Choosing which oil should be used in cooking is a big issue and concern for many people because of the fat and cholesterol contents of cooking oil. -

Natural, Vegan & Gluten-Free
Natural, Vegan & Gluten-Free All FarmHouse Fresh® products are Paraben and Sulfate free, made with up to 99.6% natural ingredients and made in Texas. Many of our products are also Vegan and Gluten-Free! Ingredient Decks Honey Heel Glaze: Water/Eau, Glycerine, Polysorbate 20, Polyquaternium-37, Parfum, Honey, Natural Rice Bran Oil (Orza Sativa), Tocopheryl Acetate (Vitamin E),Tetrahexyldecyl Ascorbate, Panthenol, Carica Papaya (Papaya) Fruit Extract, Ananas Sativus (Pineapple) Fruit Extract, Aloe Barbadensis Leaf Juice, Lactic Acid, PEG-40 Hydrogenated Castor Oil, Potassium Sorbate, Diazolidinyl Urea, DMDM Hydantoin, Caramel, Annatto. Strawberry Smash: Aloe Barbadensis Leaf Juice, Water/Eau, Glycerin, Glycine Soja (Soybean) Oil, Polysorbate 80, Butyrospermum Parkii (Shea Butter), Cetearyl Alcohol, Ceteareth-20, Cetyl Alcohol, Stearyl Alcohol, Parfum, Fragaria Vesca (Strawberry) Fruit Extract, Oryza Sativa (Rice) Barn Oil, Ethylhexyl Palmitate, Dimethicone, Cyanocobalmin (Vitamin B12), Tocopheryl Acetate, Menthone Glycerin Acetal, Carbomer, Disodium EDTA, Tetrasodium EDTA, Sodium Hydroxymethylglycinate, Sodium Hydroxide, Phenoxyethanol, Caprylyl Glycol, Potassium Sorbate Sweet Cream Body Milk: Water/Eau, Glycine Soja (Soybean) Oil, Sorbitol, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Cetyl Alcohol, Oryza Sativa (Rice) Bran Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Persea Gratissima (Avocado) Oil, Sesamum Indicum (Sesame) Seed Oil, Stearyl Alcohol, Dimethicone, Polysorbate 20, Carbomer, -

Identification of Fatty Acids in Sacha Inchi Oil (Cursive Plukenetia Volubilis L.) from Ecuador
Online - 2455-3891 Vol 11, Issue 2, 2018 Print - 0974-2441 Research Article IDENTIFICATION OF FATTY ACIDS IN SACHA INCHI OIL (CURSIVE PLUKENETIA VOLUBILIS L.) FROM ECUADOR CARRILLO W1,3*, QUINTEROS M F1, CARPIO C1, MORALES D1,VÁSQUEZ G2,ÁLVAREZ M1, SILVA M1 1Laboratory of Functional Foods, Faculty of Foods Science and Engineering, Technical University of Ambato. Av. Los Chasquis y Rio Payamino. Campus Huachi, CP 1801334, Ambato, Ecuador. 2Escuela Superior Politécnica del Litoral, ESPOL, Faculty of Mechanical Engineering and Production Sciences, Campus Gustavo Galindo Km 30.5 VíaPerimetral, Guayaquil, Ecuador. 3Department of Research. Bolivar State University, Academic Campus, Alpacha EC. Av Ernesto Che Guevara s/n and Av Gabriel Secaira, EC 020150, Guaranda, Ecuador. Email: [email protected] Received: 04 October 2016, Revised and Accepted: 10 October 2016 ABSTRACT Objective: The aim of this study was to identify fatty acids in a sacha inchi oil sample. Methods: Sacha inchi oil was obtained of sacha inchi seeds using the cold pressing method. Fatty acids analysis was carried out using the gas chromatography with a mass selective detector and using the database Library NIST14.L to identify the compounds. Results: seeds only have 3.98% of palmitic acid. Sacha inchi seeds have a high content of unsaturated fatty acids with 34.98% of ɷ6 α- Linoleic and 47.04% of ɷ3 α- Linolenic. Sacha inchi Conclusions: functional foods. Sacha inchi seed is a good source of fatty acids ɷ3 and ɷ6, being ɷ3 and ɷ6 in a good proportion. Sacha inchi oil can be used to elaborate Keywords: Pluketeniavolubilis graph Sacha inchi, , Fatty acids, Gas chromato y - mass selective detector, Methyl ester. -

Explore the Future of Baking™ with Cargill
Bakery Oils and Shortenings Application Guide www.cargill.com Global Edible Oil Solutions Explore The Future of North America PO Box 9300 ™ Minneapolis, MN 55440-9300 Baking with Cargill USA The information contained herein is believed to be true and correct under US law. All statements, recommendations or suggestions are made without guarantee, express or implied, and are subect to change without notice. We disclaim all warranties, express or implied, including any warranties of merchantability, fitness for a particular purpose and freedom from infringement and disclaim all liability in connection with the use of the products or information contained herein. © 2020 Cargill, Incorporated. All rights reserved. (03/20) Donut Puff Breads/ Pies Cake Icings Cookies Bars Danish Biscuits Pizza Tortillas Bakery Oils and Shortenings Application Guide Fry Pastry Buns Whipped Wire Crust Fillings Buttercream Stabilizers Drop or Fillings Cut All-Purpose Shortenings Regal™ All-Purpose Shortening Soybean Oil and Hydrogenated Soybean Oil or Interesterified Soybean Oil PalmAgility® 204 All-Purpose Shortening Palm Oil PalmAgility® 305 All-Purpose Shortening Palm Oil Clear Valley® All-Purpose Shortening Canola Oil, Hydrogenated Cottonseed Oil Advantage® P-100 Palm Oil Advantage® PS-102 Palm Oil, Soybean Oil Advantage® PN-110 Palm Oil, Canola Oil Advantage® P-115 Palm Oil Advantage® P-118 Palm Oil Renaissance® Lard Deodorized Lard Cake, Icing, Filling Shortenings Soybean Oil, Hydrogenated Soybean Oil, Mono and Diglycerides and Regal™ Cake and Icing Shortening -
Almond Oil 92
Iodine value Oil / Fat g/100 g Almond oil 92 - 106 94 - 101 Apricot kernel oil 97 - 110 Argan seed oil 92 - 102 Avocado oil 63 - 95 85 - 90 Babassu oil 10 - 18 10 - 18 Blackcurrent oil 173 182 Camelina oil 127 - 155 130 - 145 Cashew nut oil 79 - 89 Castor oil 82 - 88 81 - 91 Chia oil 190 - 199 196 - 199 Cocoa butter 33 - 40 32 - 40 Coconut oil 6 - 9 6,3 - 11 6 - 11 Coconut oil, cochin 7 - 12 Coconut oil, RBD 7 - 12 Corn oil 127 - 133 Cottonseed oil 103 - 115 100 - 123 100 - 115 Cottonseed oil, RBD 98 - 118 Crambe oil 93 Flaxseed oil 182 - 203 Grape seed oil 130 - 138 128 - 150 Hazelnut oil 85 - 95 Illipe butter 58 - 65 Linseed oil 170 - 204 Maize germ oil 103 - 128 103 - 135 107 - 128 Melon seed oil 124 Mustardseed oil 92 - 125 Oiticica oil 140 - 180 Iodine value Oil / Fat g/100 g Olive oil 75 - 94 Ongokea oil 180 - 205 Palm kernel oil 16 - 23 14,1 - 21 Palm oil 50,6 - 55 51 - 54 50 - 55 Palm olein 56 - 61 Palm stearin Palm oil, neutralized 50 - 55 Palm oil, neutralized and bleached 50 - 55 ≥ 56 Palm oil (RBD/NBD) 50 - 55 Palm olein, crude 56 Palm olein, neutralized 56 Palm olein, neutralized and bleached 56 Palm olein (RBD/NBD) 56 Palm stearin 22 - 49 ≤ 48 Palm superolein ≥ 60 Peanut oil 86 - 107 86 - 107 Peanut oil, Africa 85 - 90 Peanut oil, South America 92 - 110 Perilla oil 192 - 208 Poppyseed oil 130 - 143 Pumpkin seed oil 117 Rapeseed oil 94 - 120 Rapeseed oil (Canola) 110 - 126 Rapeseed oil, erucic acid incl. -

Oxidative Stability and Shelf Life of Avocado Oil Extracted Cold and Hot Using Discard Avocado (Persea Americana)
Scientia Agropecuaria 11(1): 127 – 133 (2020) SCIENTIA AGROPECUARIA Facultad de Ciencias Agropecuarias Scientia Agropecuaria Universidad Nacional de Website: http://revistas.unitru.edu.pe/index.php/scientiaagrop Trujillo Oxidative stability and shelf life of avocado oil extracted cold and hot using discard avocado (Persea americana) * Jhoseline Guillén-Sánchez ; Luz María Paucar-Menacho Universidad Nacional del Santa, Facultad de Ingeniería, Departamento de Ingeniería Agroindustrial y Agrónoma, Av. Universitaria s/n, Urb. Bellamar, Nuevo Chimbote, Ancash, Peru. Received August 31, 2019. Accepted March 8, 2020. Abstract During the last years, the world avocado trade is on the rise, however, approximately 20% of the production is rejected for low caliber. This avocado discarded by low caliber can be used in the elaboration of other products, to give it added value. The objective of this study was to determine the shelf life of Hass avocado oil, type of discard, obtained by: (a) Drying by stove/Soxhlet (b) Lyophilized/Expeller. For the physicochemical characterization of the oil, density, melting point, acidity index, refractive index, iodine index and peroxide index were determined. The induction time was carried out by the RANCIMAT method, for which three temperatures of 140 °C, 160 °C and 180 °C were used and the shelf life of the oil was obtained by extrapolation at 25 °C. The results indicate that there is a significant statistical difference in the physicochemical characteristics of both oils. The shelf life was for the oil obtained by drying stove/Soxhlet 5.94 years and for the oil obtained by freeze-dried/Expeller 4.41 years, since both oils had a high content of unsaturated fatty acids 16.98% and 17.12%, respectively.