AIDS), Was Reported in 1981 in the United States (CDC, 2006)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cornual Pregnancy Discovered on CT Scan: a Case Report Baadi F1*, Gakosso C1, Rachid2, Oubahha2, Fakhir B2, Zouita I1, Jalal H1
Scholars International Journal of Obstetrics and Gynecology Abbreviated Key Title: Sch Int J Obstet Gynec ISSN 2616-8235 (Print) |ISSN 2617-3492 (Online) Scholars Middle East Publishers, Dubai, United Arab Emirates Journal homepage: https://saudijournals.com Case Report Cornual Pregnancy Discovered on CT scan: A Case Report Baadi F1*, Gakosso C1, Rachid2, Oubahha2, Fakhir B2, Zouita I1, Jalal H1 1Radiology Department, Mother and Child Hospital, Mohammed VI CHU, Cadi Ayyad University, Marrakech 2Obstetrics and Gynecology Department, Mother and Child Hospital, Mohammed VI CHU, Cadi Ayyad University, Marrakech DOI: 10.36348/sijog.2021.v04i01.004 | Received: 26.11.2020 | Accepted: 09.12.2020 | Published: 29.01.2021 *Corresponding author: Baadi F Abstract Cornual pregnancy is uncommon among ectopic pregnancies. A diagnosis of cornual pregnancy remains challenging, and rupture of a cornual pregnancy causes catastrophic consequence due to massive bleeding. The purpose of this study is to determine the contribution of imaging in the early diagnosis and management of this rare entity, in order to avoid complications. Keywords: Cornual pregnancy, US, CT, MRI. Copyright © 2021 The Author(s): This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-NC 4.0) which permits unrestricted use, distribution, and reproduction in any medium for non-commercial use provided the original author and source are credited. NTRODUCTION This patient presents as obstetric history: fetal I death in utero estimated at 34 weeks pregnant with pre- Cornual pregnancy is a rare form of ectopic eclampsia. pregnancy, defined by the implantation of a gestational sac in the horn of the uterus, occurs in 2% of ectopic According to her gynecologist performed an pregnancies [1]. -
Terminology for Describing Normally-Sited and Ectopic Pregnancies on Ultrasound: ESHRE Recommendations for Good Practice
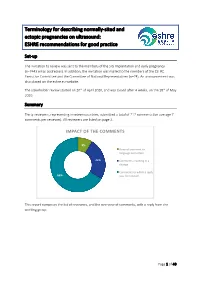
Terminology for describing normally-sited and ectopic pregnancies on ultrasound: ESHRE recommendations for good practice Set-up The invitation to review was sent to the members of the SIG Implantation and early pregnancy (n=7443 email addresses). In addition, the invitation was mailed to the members of the ESHRE Executive Committee and the Committee of National Representatives (n=74). An announcement was also placed on the eshre.eu website. The stakeholder review started on 20th of April 2020, and was closed after 4 weeks, on the 18th of May 2020. Summary Thirty reviewers, representing nineteen countries, submitted a total of 212 comments (on average 7 comments per reviewer). All reviewers are listed on page 2. IMPACT OF THE COMMENTS 9% General comment or language correction 25% Comments resulting in a change Comments to which a reply 66% was formulated This report comprises the list of reviewers, and the overview of comments, with a reply from the working group. Page 1 of 40 List of reviewers Name Country Organization Masoud Kamrava Iran Steven Goldstein USA Ulrike Metzger France Grigorios Derdelis Greece Luca Savelli Italy Gangaraju Buvaneswari India Mridu Sinha India Onur Erol Turkey Aboubakr Mohamed Elnashar Egypt Jiuzhi Zeng China Carlos Calhaz-Jorge Portugal Dr. Nidaa Qatar Attilio Di Spiezio Sardo Italy Melinda Mitranovici Romania Ali Sami Gurbuz Turkey Philippe Merviel France Roy Farquharson UK Ilan Timor USA Alessandra Pipan United Arab Emirates Company, Taiwan IVF Wen Jui Yang Taiwan Group Center Lorenzo Abad de Velasco Spain Monica Varma India Kumaran Aswathy India Annick Geril Belgium Snezana Vidakovic Serbia Lukasz Polanski, Miriam Baumgarten, Rebecca McKay, Laura Rutherford, Ouma Pillay, Anita Jeyaraj, Kanna Jayaprakasan and Kamal Ojha UK organization Mohamed Shahin UK Mohsen M El-Sayed UK International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) ISUOG European Niche taskforce group of the ESGE and international Prof. -

Sonography in First Trimester Bleeding
Review Sonography in First Trimester Bleeding Manjiri Dighe, MD,1 Carlos Cuevas, MD,1 Mariam Moshiri, MD,1 Theodore Dubinsky, MD,1 Vikram S. Dogra, MD2 1 Department of Radiology, University of Washington Medical Center, Seattle, WA 98195 2 Department of Imaging Sciences, University of Rochester School of Medicine, 601 Elmwood Avenue, Box 648, Rochester, NY 14642 Received 19 April 2007; accepted 1 October 2007 ABSTRACT: Vaginal bleeding is the most common however, the most common cause of bleeding is cause of presentation to the emergency department spotting caused by implantation of the conceptus in the first trimester. Approximately half of patients into the endometrium. A complete assessment of with first trimester vaginal bleeding will lose the preg- the first trimester pregnancy requires correlation nancy. Clinical assessment is difficult, and sonogra- of serum b human chorionic gonadotropin (b- phy is necessary to determine if a normal fetus is hCG) levels with the appearance of the gesta- present and alive and to exclude other causes of bleeding (eg, ectopic or molar pregnancy). Diagnosis tional sac (GS) using sonography. of a normal intrauterine pregnancy not only helps the physician in terms of management but also gives psy- SPONTANEOUS ABORTION chologic relief to the patient. Improved ultrasound technology and high-frequency endovaginal trans- Spontaneous abortion is defined as the termina- ducers have enabled early diagnosis of abnormal and tion of a pregnancy before the twentieth completed ectopic pregnancies, decreasing maternal morbidity week of gestation. Sixty-five percent of spontane- and mortality. The main differential considerations of ous abortions occur in the first 16 weeks of preg- first trimester bleeding are spontaneous abortion, ec- nancy. -

Ruptured Subcapsular Hematoma of the Liver Due to Pre‑Eclampsia Presenting As Interstitial Pregnancy and the Role of Intra‑Abdominal Packing
[Downloaded free from http://www.njcponline.com on Friday, February 20, 2015, IP: 41.135.175.148] || Click here to download free Android application for this journal Case Report Ruptured subcapsular hematoma of the liver due to pre‑eclampsia presenting as interstitial pregnancy and the role of intra‑abdominal packing NC Ngene, N Amin1, J Moodley2 Department of Obstetrics and Gynaecology, Edendale Hospital Pietermaritzburg, 1School of Education, 2Women’s Health and HIV Research Group, University of KwaZulu‑Natal, Durban, South Africa Abstract Ruptured subcapsular hematoma of the liver (RSHL) can mimic ruptured interstitial pregnancy because each of these conditions occasionally presents at the same gestational period and both do manifest hemodynamic instability. The similarities between the two conditions pose a diagnostic challenge, especially in an unbooked patient. We report a case of an unbooked primigravida, at 21 weeks of gestation, who arrived at a regional hospital with evidence of intra‑abdominal bleeding and hypovolemic shock. She was diagnosed as potentially having a ruptured interstitial pregnancy. During the ensuing emergency laparotomy, RSHL was discovered, the area around the ruptured liver capsule was packed with large abdominal swabs, and the patient recovered. This case report illustrates the need to consider RSHL in patients presenting with features of ruptured interstitial pregnancy, as this will assist in the planning of intraoperative care. We also describe abdominal packing and highlight the need for this essential -

Cornual Pregnancy and Interstitial Pregnancy Kornual Ve Interstisyal Gebelik
Letter to the Editor 61 Cornual pregnancy and interstitial pregnancy Kornual ve interstisyal gebelik Cemil Yaman Department of Obstetrics and Gynecology, General Hospital of Linz, Linz, Austria Dear editor; Interstitial pregnancies often progress without symptoms until a rupture occurs later than other tubal pregnancies. The man- Comments regarding the published case report from Günenç agement of cornual pregnancy can be complicated because et al “Laparoscopic surgery of interstitial (cornual) pregnancy of malformations (1, 2). Due to these facts there is a need to 2009 11-2: 102-4 in TGGA” Authors reported: “The terms cor- differentiate between these pathologies. Furthermore, the nual and interstitial pregnancy are used synonymously (page occurrence of an angular pregnancy, which is distinguished 102, line 17) to define pregnancy implanted in the interstitial from cornual and interstitial pregnancy anatomically by its part of the fallopian tube which is the proximal portion that is embodied within the muscular wall of the uterus”. position in relation to the round ligament, should be taken into the diagnostic considerations. Unlike the interstitial and Comments cornual pregnancies, angular pregnacies may have a favor- able outcome (4). In the North American literature and in a small number of European literature, the terms “interstitial pregnancy” and Conflict of interest “cornual pregnancy” are used synonymously. However, it No conflict of interest is declared by authors. seems that recent studies indicate differences between these two pregnancies (1-3). References Cornual pregnancy is a pregnancy in one horn of a bircor- nuated uterus or, by extension, in one half of a septated or 1. Malinowski A, Bates SK. -

Ectopic Pregnancy Management: Tubal and Interstitial SIGNS / SYMPTOMS DIAGNOSIS
8/29/19njm Ectopic Pregnancy Management: Tubal and interstitial SIGNS / SYMPTOMS Pain and vaginal bleeding are the hallmark symptoms of ectopic pregnancy. Pain is almost universal; it is generally lower abdominal and unilateral. Bleeding is also very common following a short period of amenorrhea. Physical exam may reveal a tender adnexal mass, often mentioned in texts, but noted clinically only 20 percent of the time. Furthermore, it may easily be confused with a tender corpus luteum of a normal intrauterine pregnancy. Finally signs and symptoms of hemoperitoneum and shock can occur, including a distended, silent, “doughy” abdomen, shoulder pain, bulging cul de sac into the posterior fornix of the vagina, and hypotension. DIAGNOSIS Initially, serum hCG rises, but then usually plateaus or falls. Transvaginal ultrasound scanning is a key diagnostic tool and can rapidly make these diagnoses: 1. Ectopic is ruled out by the presence of an intrauterine pregnancy with the exception of rare heterotopic pregnancy 2. Ectopic is proven when a gestational sac and an embryo with a heartbeat is seen outside of the uterus 3. Ectopic is highly likely if ANY adnexal mass distinct from the corpus luteum or a significant amount of free pelvic fluid is seen. When ultrasound is not definitive, correlation of serum hCG levels is important. If the hCG is above the “discriminatory zone” of 3000 mIU/ml IRP, a gestational sac should be visible on transvaginal ultrasound. The discriminatory zone varies by ultrasound machine and sonographer. If an intrauterine gestational sac is not visible by the time the hCG is at, or above, this threshold, the pregnancy has a high likelihood of being ectopic. -

Interstitial & Scar Pregnancies
Interstitial & Scar Pregnancies Jackie Ross Consultant Gynaecologist King’s College Hospital, London Declaration of financial interests I have received payment for teaching on courses in 3D ultrasound on behalf of Cook UK I have received travel expenses & meals from: Association of Early Pregnancy Units British Medical Ultrasound Society European Society of Gynaecological Endoscopists Fetal Medicine Foundation ESHRE I have received lunch from: RCOG Scope Diagnosis Diagnostic dilemmas Treatment options Interstitial ectopic pregnancy Interstitial pregnancy Clinical features 2-4% ectopic pregnancies Previous tubal ectopic/ipsilateral salpingectomy, ART, STI Delayed presentation 9-12 weeks gestation Interstitial ectopic pregnancy: diagnosis • Empty uterine cavity • Products of conception/gestational sac located laterally in the interstitial (intramural) part of the tube • Presence of the ‘ interstitial line sign’ • (Surrounded by less than 5 mm of myometrium in all imaging planes) How do you define the endometrial cavity? How do you define the endometrial cavity? Interstitial ectopic pregnancy Interstitial ectopic pregnancy Interstitial ectopic pregnancy Interstitial pregnancy Differential Diagnosis Angular pregnancy Cornual pregnancy Intramural pregnancy Intrauterine pregnancy in an anomalous uterus Fibroid Adenomyoma Interstitial ectopic pregnancy: differential diagnosis Fig. 18: MRI was performed suspecting interstitial pregnancy by TVUS at 5 weeks’ gestation. A,B. Axial (A) and coronal (B) T2-weighted MRI show a GS-like -

Sexual and Reproductive Health
Sexual and Reproductive Health Guide for the Care of the Most Relevant Obstetric Emergencies Guide for the Care of the Most Relevant Obstetric Emergencies Fescina R*, De Mucio B*, Ortiz El**, Jarquin D**. *Latin American Center for Perinatology Women and Reproductive Health **Latin American Federation of Societies of Obstetrics and Gynecology Scientific Publication CLAP/WR N° 1594-02 Latin American Center for Perinatology Women and Reproductive Health CLAP/ WR Sexual and Reproductive Health Cataloguing-in-Publication Data Fescina R, De Mucio B, Ortiz E, Jarquin D. Guide for the care of the most relevant obstetric emergencies. Montevideo: CLAP/WR; 2013. (CLAP/WR. Scientific Publication; 1594-02) ISBN: 1. Maternal Mortality - Prevention 2. Pregnancy Complications 3. Placenta Previa 4. Pre-Eclampsia 5. Pregnancy Complications, Infectious 6. Eclampsia 7. Postnatal care 8. Postpartum Hemorrhage 9. Pregnancy, High-Risk 10.Pregnancy, Ectopic I. CLAP/WR II.Title The Pan American Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and inquiries should be addressed to Editorial Services, Area of Knowledge Management and Communications (KMC), Pan American Health Organization, Washington, D.C., U.S.A. The Latin American Center for Perinatology, Women and Reproductive Health (CLAP/WR), Area of Family and Community Health, Pan American Health Organization, will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. © Pan American Health Organization, 2013 All rights reserved. Publications of the Pan American Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. -

Board-Review-Series-Obstetrics-Gynecology-Pearls.Pdf
ObstetricsandGynecology BOARDREVIEW Third Edition Stephen G. Somkuti, MD, PhD Associate Professor Department of Obstetrics and Gynecology and Reproductive Sciences Temple University School of Medicine School Philadelphia, Pennsylvania Director, The Toll Center for Reproductive Sciences Division of Reproductive Endocrinology Department of Obstetrics and Gynecology Abington Memorial Hospital Abington Reproductive Medicine Abington, Pennsylvania New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto Copyright © 2008 by the McGraw-Hill Companies, Inc. All rights reserved. Manufactured in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. 0-07-164298-6 The material in this eBook also appears in the print version of this title: 0-07-149703-X. All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such designations appear in this book, they have been printed with initial caps. McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. For more information, please contact George Hoare, Special Sales, at [email protected] or (212) 904-4069. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. -

Interstitial Pregnancy Resulting in a Viable Infant Coexistent with Massive Perivillous Fibrin Deposition: a Case Report and Literature Review
THIEME Case Report 29 Interstitial Pregnancy Resulting in a Viable Infant Coexistent with Massive Perivillous Fibrin Deposition: A Case Report and Literature Review Yusuke Tanaka, MD1 Kazuya Mimura, MD1 Takeshi Kanagawa, MD1 Masahiro Nakayama, MD, PhD2 Shinya Matsuzaki, MD, PhD1 Yukiko Kinugasa-Taniguchi, MD, PhD1 Masayuki Endo, MD, PhD1 Tadashi Kimura, MD, PhD1 1 Department of Obstetrics and Gynecology, Osaka University Address for correspondence Kazuya Mimura, MD, Department of Graduate School of Medicine, Suita, Osaka, Japan Obstetrics and Gynecology, Osaka University Graduate School of 2 Department of Pathology and Laboratory Medicine, Osaka Medical Medicine, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan Center and Research Institute for Maternal and Child Health, Izumi, (e-mail: [email protected]). Osaka, Japan Am J Perinatol Rep 2014;4:29–32. Abstract Objective The objective of this report is to describe a rare case of interstitial pregnancy ultimately resulting in a viable infant coexistent with massive perivillous fibrin deposition (MPFD). Study Design This study is a case report and literature review. Results A 35-year-old female patient underwent cesarean section at 32 weeks of gestation due to fetal growth restriction (FGR) and breech presentation. During the Keywords operation, a diagnosis of interstitial pregnancy was established. There was no evidence ► ectopic pregnancy of placental separation. We decided to complete surgery without removal of the ► fetal growth placenta and waited until the placenta delivered spontaneously. The conservative restriction management was successful, and the patient was discharged on postoperative day ► interstitial pregnancy 13. The pathologic examination showed MPFD. ► massive perivillous Conclusion If interstitial pregnancies are not diagnosed at an early gestational age, it fibrin deposition can result in a viable fetus, but such pregnancies may be associated with FGR or placenta ► placenta accreta accreta. -

Ectopic Pregnancy
Ectopic pregnancy Reviewed By Peter Chen MD, Department of Obstetrics & Gynecology, University of Pennsylvania Medical «more » Definition An ectopic pregnancy is an abnormal pregnancy that occurs outside the womb (uterus). The baby cannot survive. Alternative Names Tubal pregnancy; Cervical pregnancy; Abdominal pregnancy Causes, incidence, and risk factors An ectopic pregnancy occurs when the baby starts to develop outside the womb (uterus). The most common site for an ectopic pregnancy is within one of the tubes through which the egg passes from the ovary to the uterus (fallopian tube). However, in rare cases, ectopic pregnancies can occur in the ovary, stomach area, or cervix. An ectopic pregnancy is usually caused by a condition that blocks or slows the movement of a fertilized egg through the fallopian tube to the uterus. This may be caused by a physical blockage in the tube. Most cases are a result of scarring caused by: y Past ectopic pregnancy y Past infection in the fallopian tubes y Surgery of the fallopian tubes Up to 50% of women who have ectopic pregnancies have had swelling (inflammation) of the fallopian tubes (salpingitis) or pelvic inflammatory disease (PID). Some ectopic pregnancies can be due to: y Birth defects of the fallopian tubes y Complications of a ruptured appendix y Endometriosis y Scarring caused by previous pelvic surgery In a few cases, the cause is unknown. Sometimes, a woman will become pregnant after having her tubes tied (tubal sterilization). Ectopic pregnancies are more likely to occur 2 or more years after the procedure, rather than right after it. In the first year after sterilization, only about 6% of pregnancies will be ectopic, but most pregnancies that occur 2 - 3 years after tubal sterilization will be ectopic. -

Angular Pregnancy: a Review of Cases Reported in the Past 80 Years Michael Brett Rankin1, Allison Dunning BS2 and Elizabeth Kagan Arleo3*
Rankin et al. Obstet Gynecol cases Rev 2014, 1:3 ISSN: 2377-9004 Obstetrics and Gynaecology Cases - Reviews Review Article: Open Access Angular Pregnancy: A Review of Cases Reported in the Past 80 Years Michael Brett Rankin1, Allison Dunning BS2 and Elizabeth Kagan Arleo3* 1Department of Medicine, University of Texas at Houston, USA 2Duke Clinical Research Institute, USA 3Department of Radiology, New York Presbyterian Hospital-Weill Cornell, USA *Corresponding author: Dr. Elizabeth Kagan Arleo, New York Presbyterian Hospital-Weill Cornell, Department of Radiology, 425 East 61st Street, 9th Floor, New York 10065, USA, Tel: 212-821-0680; Fax: 212-821-0671; E-mail: [email protected] 1) Clinical presentation with painful asymmetric enlargement of Abstract the uterus, followed by Objective: To review reported cases of angular pregnancy (defined in 1898 as “implantation of the embryo just medial to the uterotubal 2) Directly observed lateral distension of the uterus, with junction, in the lateral angle of the uterine cavity” [1]) to determine or without rupture, accompanied by displacement of the round outcomes. ligament reflection laterally; Study design: A systematic search of medical electronic databases 3) Retention of the placenta in the uterine angle [2]. (1934-2013; search term: “angular pregnancy”) was performed, initially yielding 150 manuscripts; after application of selection One of the motivations for the current investigation is that despite criteria, 54 were included for review, yielding 85 cases of angular its first description abortion or vaginal delivery; nearly 120 years ago, pregnancy. Pregnancy outcomes were analyzed overall and in time much remains unknown about angular pregnancy, not only because period subgroups (period 1 = 1934-1981; period 2 = 1982-2013).