Bleeding in Pregnancy And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NEW ZEALAND OBSTETRIC ULTRASOUND GUIDELINES 2019 Iii
New Zealand Obstetric Ultrasound Guidelines 2019 Released 2019 health.govt.nz Acknowledgements The Ministry of Health appreciates the time, expertise and commitment of those involved in developing the New Zealand Obstetric Ultrasound Guidelines. Content within these guidelines is derived and modified from Canterbury District Health Board’s obstetric imaging guidelines with permission. All scan images were kindly provided by members of the clinical working group. Members of the clinical working group Dr Rachael McEwing, Radiologist (Chair) Dr David Perry, Paediatric and Obstetric Radiologist Gill Waterhouse, Sonographer Dr Pippa Kyle, Consultant Obstetrician and Maternal Fetal Medicine Specialist Rex de Ryke, Charge Sonographer Peer reviewers Dr Jay Marlow, Maternal Fetal Medicine Specialist Martin Necas, Clinical Specialist Sonographer Dr Rachel Belsham, Radiologist Dr Tom Gentles, Paediatric Cardiologist Citation: Ministry of Health. 2019. New Zealand Obstetric Ultrasound Guidelines. Wellington: Ministry of Health. Published in December 2019 by the Ministry of Health PO Box 5013, Wellington 6140, New Zealand ISBN 978-1-98-859749-2 (online) HP 7284 This document is available at health.govt.nz This work is licensed under the Creative Commons Attribution 4.0 International licence. In essence, you are free to: share ie, copy and redistribute the material in any medium or format; adapt ie, remix, transform and build upon the material. You must give appropriate credit, provide a link to the licence and indicate if changes were made. Abbreviations -

OB/GYN EMERGENCIES Elyse Watkins, Dhsc, PA-C, DFAAPA DISCLOSURES
OB/GYN EMERGENCIES Elyse Watkins, DHSc, PA-C, DFAAPA DISCLOSURES I have no financial relationships to disclose. TOPICS Ovarian torsion Postpartum hemorrhage Ruptured ectopic Acute uterine inversion pregnancy Amniotic fluid embolism Acute menorrhagia Placental abruption OVARIAN TORSION OVARIAN TORSION .Tumors (benign and malignant) are implicated in 50-60% of cases of torsion .20% occur during pregnancy (corpus luteum cyst) .Unilateral or bilateral abdominal-pelvic pain, usually sudden onset .Exercise or movement exacerbates pain .Nausea and vomiting 70% .Pathophys: reduced venous return, stromal edema, internal hemorrhage, and infarction → necrosis OVARIAN TORSION .Physical exam variable .Ultrasonography with color Doppler .Surgical referral RUPTURED ECTOPIC PREGNANCY RUPTURED ECTOPIC PREGNANCY .All patients of reproductive age with a hx of missed menses and pelvic pain should be considered to have an ectopic pregnancy until proven otherwise. .A patient with missed menses, irregular vaginal bleeding, pelvic pain, syncope, abdominal pain, and/or dizziness should be managed as a ruptured ectopic pregnancy until proven otherwise. RUPTURED ECTOPIC PREGNANCY .Physical exam of pts with a ruptured ectopic can reveal pelvic tenderness, an adnexal mass, and evidence of hemodynamic compromise. .A transvaginal ultrasound will often show an adnexal mass and/or fluid in the pouch of Douglas. .The serum qualitative βHCG will be > 5 mIu/mL. RUPTURED ECTOPIC PREGNANCY HTTPS://YOUTU.BE/TNN1FPWHOXS RUPTURED ECTOPIC PREGNANCY .Immediately order an H/H, type and cross, and place large bore IV access for fluid support. .Laparotomy is performed when patients are hemodynamically unstable or if visualization during laparoscopy was difficult. .Patients with a ruptured ectopic pregnancy must be managed emergently and surgically! ACUTE MENORRHAGIA ACUTE MENORRHAGIA .Abnormal uterine bleeding (AUB) can result in acute blood loss that causes hemodynamic compromise so prompt evaluation of vital signs is important. -

Heterotopic Pregnancy and Septic Miscarriage:A Case Report
Journal of Gynecology and Women’s Health ISSN 2474-7602 Case Report J Gynecol Women’s Health Volume 18 Issue 5 - June 2020 Copyright © All rights are reserved by Frédéric Blavier DOI: 10.19080/JGWH.2020.18.556004 Heterotopic Pregnancy and Septic Miscarriage: A Case Report Illustrating Risks of “Medical Shopping”, Mostly for Tricky Diagnoses Frédéric B1,2, Gilles F1, Danah VO1, Noëlie D3 and Leonardo G1 1Department of Obstetrics and Prenatal Medicine, UZ Brussel University Hospital, Belgium 2Department of gynecological surgery, Arnaud de Villeneuve Hospital, France 3Department of Gynecology, UZ Brussel University Hospital, Belgium Submission: May 09, 2020; Published: June 09, 2020 *Corresponding author: Frédéric Blavier, Department of Obstetrics and Prenatal Medicine, UZ Brussel University Hospital, Belgium and Department of gynecological surgery, Arnaud de Villeneuve Hospital, France Abstract Background: Spontaneous heterotopic pregnancy and septic miscarriage are both rare and, each, associated with potential severe morbidity (shock, potential infertility) and mortality. Case presentation: A young and spontaneously pregnant woman presented acute abdomen and spotting with an empty uterus, liquid in for a suspected ectopic pregnancy, were discovered ascites, and pus in the right fallopian tube. After surgery, we observed a multiple organ the Douglas and a right tube mass in ultrasound with positif hCG and moderate inflammation, without fever. During the laparoscopy performed mechanical ventilation, temporary hemodialysis and broad-spectrum antibiotherapy, the patient recovered without neurological or systemic failure (ARDS, DIC, kidneys and liver insufficiencies) with severe inflammation and several collections in the uterine wall. After hysterectomy, abortion diagnosed one week ago in a third hospital also in Brussels. The multiple organ failure, the pus in the right fallopian tube (observed duringsequelae. -

Ectopic Pregnancy
Ectopic Pregnancy P atient Information Women’s Health Service What is an Ectopic Pregnancy? Symptoms include one or more of the following; An ectopic pregnancy is where the fertilised egg Pain on one side of the lower abdomen. It implants outside the uterus (womb), most may develop sharply, or may slowly get commonly in the fallopian tube (the tube that worse over several days. It can become connects the ovary to the uterus). severe. Vaginal bleeding often occurs, but not Rarely an ectopic pregnancy can occur in the ovary, always. It is often different to the bleeding cervix or abdominal cavity. It usually occurs in the of a period. For example, the bleeding may first ten weeks of pregnancy. be heavier or lighter than a normal period. About 1 in 100 pregnancies in is ectopic. This figure The blood may look darker. However, you rises to 5 in 100 after assisted conception therapies may think the bleeding is a late period. and to 20-30 in 100 after tubal damage due to Other symptoms may occur such as infection or tubal surgery. diarrhoea, feeling faint, or pain on passing faeces (stools). What are the possible causes of an ectopic Shoulder-tip pain may develop. This is due pregnancy? to some blood leaking into the abdomen The chances of having an ectopic pregnancy can be and irritating the diaphragm (the muscle increased by the following: used to breathe). Tubal damage from pelvic infection, If the fallopian tube ruptures and causes endometriosis or appendicitis internal bleeding, you may develop severe Women who have had previous abdominal pain or ‘collapse’. -

Cornual Pregnancy Discovered on CT Scan: a Case Report Baadi F1*, Gakosso C1, Rachid2, Oubahha2, Fakhir B2, Zouita I1, Jalal H1
Scholars International Journal of Obstetrics and Gynecology Abbreviated Key Title: Sch Int J Obstet Gynec ISSN 2616-8235 (Print) |ISSN 2617-3492 (Online) Scholars Middle East Publishers, Dubai, United Arab Emirates Journal homepage: https://saudijournals.com Case Report Cornual Pregnancy Discovered on CT scan: A Case Report Baadi F1*, Gakosso C1, Rachid2, Oubahha2, Fakhir B2, Zouita I1, Jalal H1 1Radiology Department, Mother and Child Hospital, Mohammed VI CHU, Cadi Ayyad University, Marrakech 2Obstetrics and Gynecology Department, Mother and Child Hospital, Mohammed VI CHU, Cadi Ayyad University, Marrakech DOI: 10.36348/sijog.2021.v04i01.004 | Received: 26.11.2020 | Accepted: 09.12.2020 | Published: 29.01.2021 *Corresponding author: Baadi F Abstract Cornual pregnancy is uncommon among ectopic pregnancies. A diagnosis of cornual pregnancy remains challenging, and rupture of a cornual pregnancy causes catastrophic consequence due to massive bleeding. The purpose of this study is to determine the contribution of imaging in the early diagnosis and management of this rare entity, in order to avoid complications. Keywords: Cornual pregnancy, US, CT, MRI. Copyright © 2021 The Author(s): This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY-NC 4.0) which permits unrestricted use, distribution, and reproduction in any medium for non-commercial use provided the original author and source are credited. NTRODUCTION This patient presents as obstetric history: fetal I death in utero estimated at 34 weeks pregnant with pre- Cornual pregnancy is a rare form of ectopic eclampsia. pregnancy, defined by the implantation of a gestational sac in the horn of the uterus, occurs in 2% of ectopic According to her gynecologist performed an pregnancies [1]. -
Terminology for Describing Normally-Sited and Ectopic Pregnancies on Ultrasound: ESHRE Recommendations for Good Practice
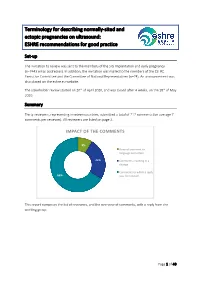
Terminology for describing normally-sited and ectopic pregnancies on ultrasound: ESHRE recommendations for good practice Set-up The invitation to review was sent to the members of the SIG Implantation and early pregnancy (n=7443 email addresses). In addition, the invitation was mailed to the members of the ESHRE Executive Committee and the Committee of National Representatives (n=74). An announcement was also placed on the eshre.eu website. The stakeholder review started on 20th of April 2020, and was closed after 4 weeks, on the 18th of May 2020. Summary Thirty reviewers, representing nineteen countries, submitted a total of 212 comments (on average 7 comments per reviewer). All reviewers are listed on page 2. IMPACT OF THE COMMENTS 9% General comment or language correction 25% Comments resulting in a change Comments to which a reply 66% was formulated This report comprises the list of reviewers, and the overview of comments, with a reply from the working group. Page 1 of 40 List of reviewers Name Country Organization Masoud Kamrava Iran Steven Goldstein USA Ulrike Metzger France Grigorios Derdelis Greece Luca Savelli Italy Gangaraju Buvaneswari India Mridu Sinha India Onur Erol Turkey Aboubakr Mohamed Elnashar Egypt Jiuzhi Zeng China Carlos Calhaz-Jorge Portugal Dr. Nidaa Qatar Attilio Di Spiezio Sardo Italy Melinda Mitranovici Romania Ali Sami Gurbuz Turkey Philippe Merviel France Roy Farquharson UK Ilan Timor USA Alessandra Pipan United Arab Emirates Company, Taiwan IVF Wen Jui Yang Taiwan Group Center Lorenzo Abad de Velasco Spain Monica Varma India Kumaran Aswathy India Annick Geril Belgium Snezana Vidakovic Serbia Lukasz Polanski, Miriam Baumgarten, Rebecca McKay, Laura Rutherford, Ouma Pillay, Anita Jeyaraj, Kanna Jayaprakasan and Kamal Ojha UK organization Mohamed Shahin UK Mohsen M El-Sayed UK International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) ISUOG European Niche taskforce group of the ESGE and international Prof. -

Sonography in First Trimester Bleeding
Review Sonography in First Trimester Bleeding Manjiri Dighe, MD,1 Carlos Cuevas, MD,1 Mariam Moshiri, MD,1 Theodore Dubinsky, MD,1 Vikram S. Dogra, MD2 1 Department of Radiology, University of Washington Medical Center, Seattle, WA 98195 2 Department of Imaging Sciences, University of Rochester School of Medicine, 601 Elmwood Avenue, Box 648, Rochester, NY 14642 Received 19 April 2007; accepted 1 October 2007 ABSTRACT: Vaginal bleeding is the most common however, the most common cause of bleeding is cause of presentation to the emergency department spotting caused by implantation of the conceptus in the first trimester. Approximately half of patients into the endometrium. A complete assessment of with first trimester vaginal bleeding will lose the preg- the first trimester pregnancy requires correlation nancy. Clinical assessment is difficult, and sonogra- of serum b human chorionic gonadotropin (b- phy is necessary to determine if a normal fetus is hCG) levels with the appearance of the gesta- present and alive and to exclude other causes of bleeding (eg, ectopic or molar pregnancy). Diagnosis tional sac (GS) using sonography. of a normal intrauterine pregnancy not only helps the physician in terms of management but also gives psy- SPONTANEOUS ABORTION chologic relief to the patient. Improved ultrasound technology and high-frequency endovaginal trans- Spontaneous abortion is defined as the termina- ducers have enabled early diagnosis of abnormal and tion of a pregnancy before the twentieth completed ectopic pregnancies, decreasing maternal morbidity week of gestation. Sixty-five percent of spontane- and mortality. The main differential considerations of ous abortions occur in the first 16 weeks of preg- first trimester bleeding are spontaneous abortion, ec- nancy. -

Ruptured Subcapsular Hematoma of the Liver Due to Pre‑Eclampsia Presenting As Interstitial Pregnancy and the Role of Intra‑Abdominal Packing
[Downloaded free from http://www.njcponline.com on Friday, February 20, 2015, IP: 41.135.175.148] || Click here to download free Android application for this journal Case Report Ruptured subcapsular hematoma of the liver due to pre‑eclampsia presenting as interstitial pregnancy and the role of intra‑abdominal packing NC Ngene, N Amin1, J Moodley2 Department of Obstetrics and Gynaecology, Edendale Hospital Pietermaritzburg, 1School of Education, 2Women’s Health and HIV Research Group, University of KwaZulu‑Natal, Durban, South Africa Abstract Ruptured subcapsular hematoma of the liver (RSHL) can mimic ruptured interstitial pregnancy because each of these conditions occasionally presents at the same gestational period and both do manifest hemodynamic instability. The similarities between the two conditions pose a diagnostic challenge, especially in an unbooked patient. We report a case of an unbooked primigravida, at 21 weeks of gestation, who arrived at a regional hospital with evidence of intra‑abdominal bleeding and hypovolemic shock. She was diagnosed as potentially having a ruptured interstitial pregnancy. During the ensuing emergency laparotomy, RSHL was discovered, the area around the ruptured liver capsule was packed with large abdominal swabs, and the patient recovered. This case report illustrates the need to consider RSHL in patients presenting with features of ruptured interstitial pregnancy, as this will assist in the planning of intraoperative care. We also describe abdominal packing and highlight the need for this essential -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME NORIDAY® 28 DAY 0.35 mg tablets .2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 0.35 mg of norethisterone. Excipients with known effect • Lactose monohydrate For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet White, round, flat with bevelled edges, 7/32” diameter, inscribed “SEARLE” on one side and “NY” on the other. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications For oral contraception in women for whom estrogens may not be appropriate. 4.2 Dose and method of administration To achieve maximum contraceptive effectiveness, NORIDAY 28 DAY must be taken exactly as directed. One tablet is taken every day at the same time, with no interruption, whether bleeding occurs or not. Each subsequent pack is started on the day after the previous pack is finished. Contraceptive efficacy may be reduced if a tablet is taken more than 3 hours late. To provide added protection during the first cycle only, the patient should be instructed to use an additional method of contraception (with the exception of rhythm, temperature and cervical- mucus methods). How to start NORIDAY 28 DAY No preceding hormonal contraceptive use (in the past month) Tablet-taking should start on the FIRST DAY of menstrual bleeding. If starting on another day, a non-hormonal back-up method of birth control (such as condoms and spermicide) should be used for the first 48 hours. Thereafter, one tablet is taken continuously at the same time every day preferably in the early evening even during menstrual bleeding. Version: pfdnorit10118 Supercedes: pfdnorit10705 Page 1 of 12 Changing from another type of progestin-only method (implant, injection) Tablet-taking should start on the day of an implant removal or, if using an injection, the day the next injection would be due. -

Heterotopic Pregnancy: Challenges in Diagnosis and Management
Open Journal of Obstetrics and Gynecology, 2016, 6, 445-450 Published Online July 2016 in SciRes. http://www.scirp.org/journal/ojog http://dx.doi.org/10.4236/ojog.2016.68059 Heterotopic Pregnancy: Challenges in Diagnosis and Management V. Sunita*, M. Ramya, T. Shruthi, G. Sangeetha Department of Obstetrics and Gynaecology, Sri Devraj Urs Medical College, Kolar, India Received 21 March 2016; accepted 20 June 2016; published 23 June 2016 Copyright © 2016 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Objectives: 1) To study the obstetric outcome in two women with heterotopic pregnancy, 2) To report the challenges faced during management of these pregnancies. Design: Case study of two heterotopic pregnancies managed by us. Setting: RL Jalappa Hospital and Research Hospital. Pa- tients: Two women with heterotopic pregnancy. Interventions: Laparotomy and management of ectopic component. Main Outcome Measures: Pregnancy outcomes following surgery. Results: Both pregnancies continued with one live birth at 36 weeks and one preterm delivery at 29 weeks with neonatal death subsequently. Conclusion: Pregnancy outcome after surgery for heterotopic pregnancy is guarded and is at high risk for preterm birth. Salpingostomy is a good option when faced with dilemma of whether it is hematosalpinx or ectopic pregnancy instead of salpingect- omy. Keywords Heterotopic Pregnancy, Hematosalpinx, Ectopic Pregnancy 1. Introduction Heterotopic pregnancy is a rare situation where intrauterine and extra uterine pregnancy occur simultaneously. Incidence in general population is around 1:30,000 for a naturally conceived pregnancy [1]. -

Cornual Pregnancy and Interstitial Pregnancy Kornual Ve Interstisyal Gebelik
Letter to the Editor 61 Cornual pregnancy and interstitial pregnancy Kornual ve interstisyal gebelik Cemil Yaman Department of Obstetrics and Gynecology, General Hospital of Linz, Linz, Austria Dear editor; Interstitial pregnancies often progress without symptoms until a rupture occurs later than other tubal pregnancies. The man- Comments regarding the published case report from Günenç agement of cornual pregnancy can be complicated because et al “Laparoscopic surgery of interstitial (cornual) pregnancy of malformations (1, 2). Due to these facts there is a need to 2009 11-2: 102-4 in TGGA” Authors reported: “The terms cor- differentiate between these pathologies. Furthermore, the nual and interstitial pregnancy are used synonymously (page occurrence of an angular pregnancy, which is distinguished 102, line 17) to define pregnancy implanted in the interstitial from cornual and interstitial pregnancy anatomically by its part of the fallopian tube which is the proximal portion that is embodied within the muscular wall of the uterus”. position in relation to the round ligament, should be taken into the diagnostic considerations. Unlike the interstitial and Comments cornual pregnancies, angular pregnacies may have a favor- able outcome (4). In the North American literature and in a small number of European literature, the terms “interstitial pregnancy” and Conflict of interest “cornual pregnancy” are used synonymously. However, it No conflict of interest is declared by authors. seems that recent studies indicate differences between these two pregnancies (1-3). References Cornual pregnancy is a pregnancy in one horn of a bircor- nuated uterus or, by extension, in one half of a septated or 1. Malinowski A, Bates SK. -

A B C Pregnancy Terms and Definitions
Pregnancy Terms and Definitions Obstetrics & Gynecology A After pains or afterbirth pains: Contractions of the uterus that occur after your baby is born, as the uterus returns to its normal size. This may cause cramping for a few days, especially if this is not your first baby or if you are nursing. Amniocentesis: the removal of a sample of amniotic fluid by means of a needle inserted through the mother’s abdominal wall; used for genetic and biochemical analysis of the baby. Amniotic fluid: the liquid surrounding and protecting the baby within the amniotic sac throughout pregnancy. Amniotic sac: the membrane within the uterus that contains the baby and the amniotic fluid. Analgesic: Medication that relieves or reduces pain. Anesthesia: Loss of feeling. There are three ways of doing this: general, local and epidural. Anesthesiologist: A doctor who specializes in the use of anesthesia. Anesthetist: A registered nurse who has special training in anesthesia. Apgar score rating: A system to evaluate the health of your baby immediately after birth. The score can be zero to 10, based on appearance and color, pulse, reflexes, activity and respiration. B Baby blues: A mild depression many women feel in the first few weeks after birth. Braxton-Hicks contractions: Mild, usually painless contractions that occur during the entire pregnancy, but are only felt from the 5th month on. Breech birth: Baby is born feet or buttocks first. C Cephalopelvic disproprition (CPD): Baby’s head is too large for the mother’s pelvic bones. Cervix: the neck of the uterus; Pap smears are taken from the cervix.