Resistance Et Évolution Des Poux Humains Pediculus Humanus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reporting of Diseases and Conditions Regulation, Amendment, M.R. 289/2014
THE PUBLIC HEALTH ACT LOI SUR LA SANTÉ PUBLIQUE (C.C.S.M. c. P210) (c. P210 de la C.P.L.M.) Reporting of Diseases and Conditions Règlement modifiant le Règlement sur la Regulation, amendment déclaration de maladies et d'affections Regulation 289/2014 Règlement 289/2014 Registered December 23, 2014 Date d'enregistrement : le 23 décembre 2014 Manitoba Regulation 37/2009 amended Modification du R.M. 37/2009 1 The Reporting of Diseases and 1 Le présent règlement modifie le Conditions Regulation , Manitoba Règlement sur la déclaration de maladies et Regulation 37/2009, is amended by this d'affections , R.M. 37/2009. regulation. 2 Schedules A and B are replaced with 2 Les annexes A et B sont remplacées Schedules A and B to this regulation. par les annexes A et B du présent règlement. Coming into force Entrée en vigueur 3 This regulation comes into force on 3 Le présent règlement entre en vigueur January 1, 2015, or on the day it is registered le 1 er janvier 2015 ou à la date de son under The Statutes and Regulations Act , enregistrement en vertu de Loi sur les textes whichever is later. législatifs et réglementaires , si cette date est postérieure. December 19, 2014 Minister of Health/La ministre de la Santé, 19 décembre 2014 Sharon Blady 1 SCHEDULE A (Section 1) 1 The following diseases are diseases requiring contact notification in accordance with the disease-specific protocol. Common name Scientific or technical name of disease or its infectious agent Chancroid Haemophilus ducreyi Chlamydia Chlamydia trachomatis (including Lymphogranuloma venereum (LGV) serovars) Gonorrhea Neisseria gonorrhoeae HIV Human immunodeficiency virus Syphilis Treponema pallidum subspecies pallidum Tuberculosis Mycobacterium tuberculosis Mycobacterium africanum Mycobacterium canetti Mycobacterium caprae Mycobacterium microti Mycobacterium pinnipedii Mycobacterium bovis (excluding M. -

Especial Número 1
TIEMPO Y SOCIEDAD Revista de Historia y Humanidades <http://tiemposociedad.wordpress.com> Núm Especial 1: Septiembre 2009-Abril 2013 1 Dirección y contacto Isabel López Fernández Consejo Editorial Javier Bayón Iglesias (Licenciado en Historia); Miguel Ángel Domínguez Pérez (Licenciado en Historia); Maite Valdés Blanco (Licenciada en Historia del Arte); Miguel Menéndez Méndez (Licenciado en Historia. DEA en Historia Moderna); Serafín Bodelón García (Catedrático. Doctor en Filosofía y Letras, Sección Filología Clásica); Luis Casteleiro Oliveros (Filólogo); Mauricio Díaz Rodríguez (Licenciado en Historia); Pablo Folgueira Lombardero (Licenciado en Historia. DEA en Arqueología) Tiempo y Sociedad. Revista de Historia y Humanidades Editora: Isabel López Fernández Portada y Logotipo: José Manuel Muñoz Fernández ISSN: 1989-6883 Tiempo y Sociedad no se hace responsable de las opiniones vertidas por los autores en sus artículos, que serán responsabilidad exclusiva de dichos autores. Esta publicación se distribuye bajo licencia Creative Commons. Está permitida su libre descarga, difusión y reproducción. Solo se han de tomar las debidas medidas de citación y referenciación. Oppidum Noega 2013 2 Índex Introducción………………………………………………………………………………………………5 Espacio global y tiempo profundo, por Josep Fontana…………………………………7 The modelling skulls in the Ancient Near-East, por Florine Marchand……………………………………………………………………………………………………………..21 Primeros siglos de cristianismo en Asturias, por Narciso Santos Yanguas…………………………………………………………………………………………………………………59 La estrategia exterior de Castiella vista desde Francia, por Álvaro Fleites Marcos…………………………………………………………………………………………………………………….113 Cien años de Historia del Arte en España, por Gonzalo M. Borrás Gualis…………………………………………………………………………………………………………….…133 3 Introducción Parece que fue ayer cuando tres amigos se reunieron en casa de uno de ellos para empezar a hablar de lo que ha terminado siendo Tiempo y Sociedad, y ya han pasado más de tres años y diez números. -

Download Full Article in PDF Format
Hafting and raw materials from animals. Guide to the identification of hafting traces on stone tools Veerle ROTS Prehistoric Archaeology Unit Katholieke Universiteit Leuven Geo-Institute Celestijnenlaan 200E (Pb: 02409), B-3001 Leuven, Heverlee (Belgique) [email protected] Rots V. 2008. – Hafting and raw materials from animals. Guide to the identification of hafting traces on stone tools. [DVD-ROM]1 . Anthropozoologica 43 (1): 43-66. ABSTRACT Stone tool hafting has been a widely discussed topic, but its identifica- tion on a prehistoric level has long been hampered. Given the organic nature of hafting arrangements, few remains are generally preserved. An overview is presented of animal materials that can be used for haft- ing stone tools, and examples are provided of preserved hafting arrangements made out of animal raw material. Based on the same principles as those determining the formation of use-wear traces on stone tools, it is argued that hafting traces are formed and can be iden- tified. The variables influencing the formation of hafting traces are KEY WORDS discussed. Specific wear patterns and trace attributes are provided for Stone tools, use-wear, different hafting arrangements that use animal raw material. It is hafting, concluded that the provided referential data allow for the identifi- wear pattern, experiments, cation of hafted stone tools on prehistoric sites and the identification animal raw material. of the hafting arrangement used. RÉSUMÉ Emmanchements et matières premières animales. Un guide pour l’identification des traces d’emmanchement sur des outils de pierre. Le sujet des emmanchements des outils de pierre a été largement discuté, mais leurs identifications à un niveau préhistorique ont longtemps été difficiles. -

Diversitat De Comunitats Heteròtrofes Associades a Les Aigües De Consum
Diversitat de comunitats heteròtrofes associades a les aigües de consum Laura Sala Comorera ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tdx.cat) i a través del Dipòsit Digital de la UB (diposit.ub.edu) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX ni al Dipòsit Digital de la UB. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX o al Dipòsit Digital de la UB (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tdx.cat) y a través del Repositorio Digital de la UB (diposit.ub.edu) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. No se autoriza su reproducción con finalidades de lucro ni su difusión y puesta a disposición desde un sitio ajeno al servicio TDR o al Repositorio Digital de la UB. -

Reportable Disease Surveillance in Virginia, 2013
Reportable Disease Surveillance in Virginia, 2013 Marissa J. Levine, MD, MPH State Health Commissioner Report Production Team: Division of Surveillance and Investigation, Division of Disease Prevention, Division of Environmental Epidemiology, and Division of Immunization Virginia Department of Health Post Office Box 2448 Richmond, Virginia 23218 www.vdh.virginia.gov ACKNOWLEDGEMENT In addition to the employees of the work units listed below, the Office of Epidemiology would like to acknowledge the contributions of all those engaged in disease surveillance and control activities across the state throughout the year. We appreciate the commitment to public health of all epidemiology staff in local and district health departments and the Regional and Central Offices, as well as the conscientious work of nurses, environmental health specialists, infection preventionists, physicians, laboratory staff, and administrators. These persons report or manage disease surveillance data on an ongoing basis and diligently strive to control morbidity in Virginia. This report would not be possible without the efforts of all those who collect and follow up on morbidity reports. Divisions in the Virginia Department of Health Office of Epidemiology Disease Prevention Telephone: 804-864-7964 Environmental Epidemiology Telephone: 804-864-8182 Immunization Telephone: 804-864-8055 Surveillance and Investigation Telephone: 804-864-8141 TABLE OF CONTENTS INTRODUCTION Introduction ......................................................................................................................................1 -

David L.Reed
Curriculum Vitae David L. Reed DAVID L. REED (February 2021) Associate Provost University of Florida EDUCATION AND PROFESSIONAL DEVELOPMENT: Management Development Program, Graduate School of Education, Harvard Univ. 2016 Advanced Leadership for Academic Professionals, University of Florida 2016 Ph.D. Louisiana State University (Biological Sciences) 2000 M.S. Louisiana State University (Zoology) 1994 B.S. University of North Carolina, Wilmington (Biological Sciences) 1991 PROFESSIONAL EXERIENCE: Administrative (University of Florida) Associate Provost, Office of the Provost 2018 –present Associate Director for Research and Collections, Florida Museum 2015 – 2020 Provost Fellow, Office of the Provost 2017 – 2018 Assistant Director for Research and Collections, Florida Museum 2012 – 2015 Academic Curator of Mammals, Florida Museum, University of Florida 2014 – present Associate Curator of Mammals, Florida Museum, University of Florida 2009 – 2014 Assistant Curator of Mammals, Florida Museum, University of Florida 2004 – 2009 Research Assistant Professor, Department of Biology, University of Utah 2003 – 2004 NSF Postdoctoral Fellow, Department of Biology, University of Utah 2001 – 2003 Courtesy/Adjunct Graduate Faculty, Department of Wildlife Ecology and Conservation, UF 2009 – present Graduate Faculty, School of Natural Resources and the Environment, UF 2005 – present Graduate Faculty, Genetics and Genomics Graduate Program, UF 2005 – present Graduate Faculty, Department of Biology, UF 2004 – present ADMINISTRATIVE RECORD: Associate Provost, Office of the Provost, University of Florida July 2018-present Artificial Intelligence • Serve on and organize the AI Executive Workgroup dedicated to highest priority aspects of the AI Initiative. • Organize and host (Emcee) the all-day AI Retreat in April 2020. Had over 600 participants. • Establish AI Workgroups on nearly a dozen topics • Establish and Chair the AI Academic Workgroup focused on the AI Certificate, new and existing AI courses, the development of new certificates, minors, tracks and majors. -

Epidemiological and Molecular Analysis of Virulence and Antibiotic Resistance in Acinetobacter Baumannii
UNIVERSIDAD COMPLUTENSE DE MADRID FACULTAD DE VETERINARIA DEPARTAMENTO DE BIOQUÍMICA Y BIOLOGÍA MOLECULAR IV TESIS DOCTORAL Epidemiological and Molecular Analysis of Virulence and Antibiotic Resistance in Acinetobacter baumannii Análisis Epidemiológico y Molecular de la Virulencia y la Antibiorresistencia en Acinetobacter baumannii MEMORIA PARA OPTAR AL GRADO DE DOCTOR PRESENTADA POR Elias Dahdouh DIRECTORA Mónica Suárez Rodríguez Madrid, 2017 © Elias Dahdouh, 2016 UNIVERSIDAD COMPLUTENSE DE MADRID FACULTAD DE VETERINARIA DEPARTAMENTO DE BIOQUIMICA Y BIOLOGIA MOLECULAR IV TESIS DOCTORAL Análisis Epidemiológico y Molecular de la Virulencia y la Antibiorresistencia en Acinetobacter baumannii Epidemiological and Molecular Analysis of Virulence and Antibiotic Resistance in Acinetobacter baumannii MEMORIA PARA OPTAR AL GRADO DE DOCTOR PRESENTADA POR Elias Dahdouh Directora Mónica Suárez Rodríguez Madrid, 2016 UNIVERSIDAD COMPLUTENSE DE MADRID FACULTAD DE VETERINARIA Departamento de Bioquímica y Biología Molecular IV ANALYSIS EPIDEMIOLOGICO Y MOLECULAR DE LA VIRULENCIA Y LA ANTIBIORRESISTENCIA EN Acinetobacter baumannii EPIDEMIOLOGICAL AND MOLECULAR ANALYSIS OF VIRULENCE AND ANTIBIOTIC RESISTANCE IN Acinetobacter baumannii MEMORIA PARA OPTAR AL GRADO DE DOCTOR PRESENTADA POR Elias Dahdouh Bajo la dirección de la doctora Mónica Suárez Rodríguez Madrid, Diciembre de 2016 First and foremost, I would like to thank God for the continued strength and determination that He has given me. I would also like to thank my father Abdo, my brother Charbel, my fiancée, Marisa, and all my friends for their endless support and for standing by me at all times. Moreover, I would like to thank Dra. Monica Suarez Rodriguez and Dr. Ziad Daoud for giving me the opportunity to complete this doctoral study and for their guidance, encouragement, and friendship. -

Molecular Survey of the Head Louse Pediculus Humanus Capitis in Thailand and Its Potential Role for Transmitting Acinetobacter Spp
Sunantaraporn et al. Parasites & Vectors (2015) 8:127 DOI 10.1186/s13071-015-0742-4 RESEARCH Open Access Molecular survey of the head louse Pediculus humanus capitis in Thailand and its potential role for transmitting Acinetobacter spp. Sakone Sunantaraporn1, Vivornpun Sanprasert2, Theerakamol Pengsakul3, Atchara Phumee2, Rungfar Boonserm2, Apiwat Tawatsin4, Usavadee Thavara4 and Padet Siriyasatien2,5* Abstract Background: Head louse infestation, which is caused by Pediculus humanus capitis, occurs throughout the world. With the advent of molecular techniques, head lice have been classified into three clades. Recent reports have demonstrated that pathogenic organisms could be found in head lice. Head lice and their pathogenic bacteria in Thailand have never been investigated. In this study, we determined the genetic diversity of head lice collected from various areas of Thailand and demonstrated the presence of Acinetobacter spp. in head lice. Methods: Total DNA was extracted from 275 head louse samples that were collected from several geographic regions of Thailand. PCR was used to amplify the head louse COI gene and for detection of Bartonella spp. and Acinetobacter spp. The amplified PCR amplicons were cloned and sequenced. The DNA sequences were analyzed via the neighbor-joining method using Kimura’s 2-parameter model. Results: The phylogenetic tree based on the COI gene revealed that head lice in Thailand are clearly classified into two clades (A and C). Bartonella spp. was not detected in all the samples, whereas Acinetobacter spp. was detected in 10 samples (3.62%), which consisted of A. baumannii (1.45%), A. radioresistens (1.45%), and A. schindleri (0.72%). The relationship of Acinetobacter spp. -
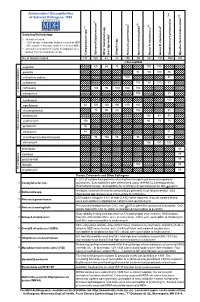
Antimicrobial Susceptibilities of Selected Pathogens, 1999
✔ Antimicrobial Susceptibilities * * † 7 of Selected Pathogens, 1999 8 e † a 2 ✔ ✔ † 3 ✔ † 4 5 6 culosis * r 1 urium spp. m L spp. Sampling Methodology 2 L † all isolates tested * ~ 20% sample of statewide isolates received at MDH spp. ~10% sample of statewide isolates received at MDH Salmonella ** all isolates tested from 7-county metropolitan area oup A streptococci ✔ oup B streptococci r isolates from a normally sterile site r Other (non-typhoidal) G Campylobacter Salmonella typhi Shigella Neisseria gonorrhoeae Neisseria meningitidis G Streptococcus pneumoni Mycobacterium tube No. of Isolates Tested 131 160 43 20 250 55 162 192 559 163 123456 123456% Susceptib123456le 123456123456 123456 123451623456 123451623456 ampicillin 1234561234566012345686 123456 15123456123456 100 100 123456123456 123451623451623451623451623456 123456 penicillin 123456123456123456123456123456 98100 100 76 123456 123456123456123456123456123456123456123456123456 123456 123451623451623451623456 123451623451623456 123456 cefuroxime sodium 123456123456123456123456 100123456123456123456 81 123456 123456123456123456123456123456123456123456123456 123456 cefotaxime 123451623451623451623451623456 100 100100 83 123456 123456123456123456123456123456 123456123456123456123456 123456 123456123456123456123456 ceftriaxone 123456 100 95 100 100 100 123451623451623451623456 -lactam antibiotics 123456123456123456123456123456 123456123456123456123456 β 123451623451623451623451623456 123451623456 123456 meropenem 123456123456123456123456123456 100 123456123456 83 123456 123456123456123456123456123456123456123456123456 -

Microbial NAD Metabolism: Lessons from Comparative Genomics
Dartmouth College Dartmouth Digital Commons Dartmouth Scholarship Faculty Work 9-2009 Microbial NAD Metabolism: Lessons from Comparative Genomics Francesca Gazzaniga Rebecca Stebbins Sheila Z. Chang Mark A. McPeek Dartmouth College Charles Brenner Carver College of Medicine Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Biochemistry Commons, Genetics and Genomics Commons, Medicine and Health Sciences Commons, and the Microbiology Commons Dartmouth Digital Commons Citation Gazzaniga, Francesca; Stebbins, Rebecca; Chang, Sheila Z.; McPeek, Mark A.; and Brenner, Charles, "Microbial NAD Metabolism: Lessons from Comparative Genomics" (2009). Dartmouth Scholarship. 1191. https://digitalcommons.dartmouth.edu/facoa/1191 This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Dartmouth Scholarship by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. MICROBIOLOGY AND MOLECULAR BIOLOGY REVIEWS, Sept. 2009, p. 529–541 Vol. 73, No. 3 1092-2172/09/$08.00ϩ0 doi:10.1128/MMBR.00042-08 Copyright © 2009, American Society for Microbiology. All Rights Reserved. Microbial NAD Metabolism: Lessons from Comparative Genomics Francesca Gazzaniga,1,2 Rebecca Stebbins,1,2 Sheila Z. Chang,1,2 Mark A. McPeek,2 and Charles Brenner1,3* Departments of Genetics and Biochemistry and Norris Cotton Cancer Center, Dartmouth Medical School, Lebanon, New Hampshire -

Primary Sources of Archaeobotanical Datasets and Radiocarbon Dates from Southwest Asia
Asouti, Eleni, and Dorian Q Fuller. A Contextual Approach to the Emergence of Agriculture in Southwest Asia: Reconstructing Early Neolithic Plant-Food Production. Supplement A: Primary Sources of Archaeobotanical Datasets and Radiocarbon Dates from Southwest Asia. Current Anthropology 54(3). Supplement A: Primary Sources of Archaeobotanical Datasets and Radiocarbon Dates from Southwest Asia We have assembled in this supplement the primary sources of archaeobotanical datasets retrieved from sites in Southwest Asia (Part 1) and the radiocarbon dates by which these sites have been assigned calendrical ages (Part 2). Part 3 presents the radiocarbon dates for early PPN sites that, to date, have not produced relevant published archaeobotanical assemblages. Part 1: Sites with Published Archaeobotanical Datasets For general overviews see Asouti and Fuller (2012), Charles (2007), Colledge and Conolly (2007), Colledge, Conolly, and Shennan (2004), Fuller et al. (2012), Garrard (1999), Willcox (1999, 2007) and Zohary, Hopf, and Weiss (2012). Sources for individual sites are indicated below, listed alphabetically by site name, followed by a short assessment of plant domestication status for all sites listed in Tables 3-4, and a list of bibliographic references. 1A. Archaeobotanical primary data sources Abdul Hosein, Tepe Iran Hubbard 1990. ‘Abr, Tell Syria Willcox et al. 2009, Willcox et al. 2008. Abu Hureyra, Tell Syria Colledge and Conolly 2010; Hillman 2000; Hillman et al. 1989; Hillman et al. 2001; de Moulins 1997, 2000. ‘Ain Ghazal Jordan Rollefson et al. 1985. Ali Kosh Iran Helbaek 1969. Aşıklı Höyük Turkey van Zeist and de Roller 1995. Aswad, Tell Syria van Zeist and Bakker-Heeres 1985 [1982]. Ayios Epiktitos Vrysi Cyprus Kyllo 1982. -

Clinical Report: Head Lice
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Head Lice Cynthia D. Devore, MD, FAAP, Gordon E. Schutze, MD, FAAP, THE COUNCIL ON SCHOOL HEALTH AND COMMITTEE ON INFECTIOUS DISEASES Head lice infestation is associated with limited morbidity but causes a high abstract level of anxiety among parents of school-aged children. Since the 2010 clinical report on head lice was published by the American Academy of Pediatrics, newer medications have been approved for the treatment of head lice. This revised clinical report clarifies current diagnosis and treatment protocols and provides guidance for the management of children with head lice in the school setting. Head lice (Pediculus humanus capitis) have been companions of the human species since antiquity. Anecdotal reports from the 1990s estimated annual direct and indirect costs totaling $367 million, including remedies and other consumer costs, lost wages, and school system expenses. More recently, treatment costs have been estimated at $1 billion.1 It is important to note that head lice are not a health hazard or a sign of poor hygiene and This document is copyrighted and is property of the American Academy of Pediatrics and its Board of Directors. All authors have filed are not responsible for the spread of any disease. Despite this knowledge, conflict of interest statements with the American Academy of there is significant stigma resulting from head lice infestations in many Pediatrics. Any conflicts have been resolved through a process approved by the Board of Directors. The American Academy of developed countries, resulting in children being ostracized from their Pediatrics has neither solicited nor accepted any commercial schools, friends, and other social events.2,3 involvement in the development of the content of this publication.