Standard Operating Procedures for Laboratory Services
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modelling of Red Blood Cell Morphological and Deformability Changes During In-Vitro Storage
applied sciences Article Modelling of Red Blood Cell Morphological and Deformability Changes during In-Vitro Storage Nadeeshani Geekiyanage 1 , Emilie Sauret 1,*, Suvash Saha 2 , Robert Flower 3 and YuanTong Gu 1 1 School of Mechanical, Medical and Process Engineering, Science and Engineering Faculty, Queensland University of Technology (QUT), Brisbane City, QLD 4000, Australia; [email protected] (N.G.); [email protected] (Y.G.) 2 School of Mechanical and Mechatronic Engineering, University of Technology Sydney (UTS), Ultimo, NSW 2007, Australia; [email protected] 3 Research and Development, Australian Red Cross Lifeblood, Kelvin Grove, QLD 4059, Australia; [email protected] * Correspondence: [email protected] Received: 28 February 2020; Accepted: 27 April 2020; Published: 4 May 2020 Featured Application: Red blood cell (RBC) storage lesion is a critical issue facing transfusion treatments, and significant changes in RBC morphology and deformability are observed due to the storage lesion. RBCs require high deformability to sustain in-vivo circulation, and impaired deformability leads to several post-transfusion adverse outcomes. Therefore, improved understanding of the interrelation between the morphological and deformability changes and the quality and viability of the stored RBCs is essential to prevent or reduce the transfusion related adverse outcomes. To support this requisite, the influence on RBC deformability due to several aspects of the storage lesion, namely, the changes in cell morphology, surface area and volume, RBC membrane biomechanics, and cytoskeletal structural integrity are explored numerically in this study. Abstract: Storage lesion is a critical issue facing transfusion treatments, and it adversely affects the quality and viability of stored red blood cells (RBCs). -

Detection of Anemia Disease Using Pso Algorithm and Lbp Texture Analysis
International Journal of Pure and Applied Mathematics Volume 120 No. 6 2018, 15-26 ISSN: 1314-3395 (on-line version) url: http://www.acadpubl.eu/hub/ Special Issue http://www.acadpubl.eu/hub/ DETECTION OF ANEMIA DISEASE USING PSO ALGORITHM AND LBP TEXTURE ANALYSIS 1S. Dhanasekaran M.E., 2Dr. N. R. Shanker Ph.D., 1Research Scholar, 2Professor/ Supervisor-Aalim Muhammed Salegh College of Engineering Department of Electronics and Communication Engineering PRIST University, Thanjavur, Tamilnadu Abstract: Nowadays, patients with anemia disease oxygen from the lungs to different parts of the body and present in the world increased by around 60-70% also to carrying maximum carbon dioxide (CO2) from respectively. The digital image processing technique has different parts of the body to lungs. successfully characterised to introduce new methods for Functional near-infrared spectroscopy (fNIRS) is disease analysis has lead to reliable systems and more utilised to differentiatethe patient with schizophrenia, and accurate for anemia disease diagnosis. This paper gives the healthy persons are based on the support vector an algorithm for the automatic detection of anemia machine (SVM) and principal component analysis disease through palm image. For solving such issues, a (PCA). Firstly, PCA is utilized to select the features on PSO algorithm and LBP texture analysis are applied for oxygenated haemoglobin (oxy-Hb) signals from the classification of palm images. There are several features different channel fNIRS data. Secondly, aextraction is are consider based on statistical analysis, i.e. mean, based on SVM is planned to separate the schizophrenia variance and entropy have been extracted. The from a healthy people. -

Clinical Hematology 1
CLINICAL HEMATOLOGY 1 CLINICAL HEMATOLOGY Editor Gamal Abdul Hamid, MD,PhD Associate Professor Faculty of Medicine and Health Sciences University of Aden CLINICAL HEMATOLOGY 2 PREFACE Clinical Hematology, first edition is written specifically for medical students, the clinician and resident doctors in training and general practioner. It is a practical guide to the diagnosis and treatment of the most common disorders of red blood cells, white blood cells, hemostasis and blood transfusion medicine. Each disease state is discussed in terms of the pathophysiology, clinical and paraclinical features which support the diagnosis and differential diagnosis. We bring together facts, concepts, and protocols important for the practice of hematology. In addition this book is also supported with review questions and quizzes. G.A-H 2012 CLINICAL HEMATOLOGY 3 CONTENTS Preface 1. Hematopoiesis 7 2. Anemia 26 3. Iron Deficiency Anemia 32 4. Hemolytic Anemia 41 5. Sickle Cell Hemoglobinopathies 49 6. Thalassemia 57 7. Hereditary Hemolytic Anemia 63 8. Acquired Hemolytic Anemia 68 9. Macrocytic Anemia 75 10. Bone Marrow Failure, Panctopenia 87 11. Spleen 95 12. Acute Leukemia 99 13. Chronic Myeloproliferative Disorders 125 14. Chronic Lymphoproliferative Disorders 137 15. Malignant Lymphoma 147 16. Multiple Myeloma and Related Paraproteinemia 171 17. Hemorrhagic Diseases 179 18. Transfusion Medicine 201 19. Bone Marrow Transplantations 214 CLINICAL HEMATOLOGY 4 Appendices: I. Hematological Tests and Normal Values 221 II. CD Nomenclature for Leukocytes Antigen 226 III. Cytotoxic Drugs 228 IV. Drugs Used in Hematology 230 Glossary 232 Answers 246 Bibliography 247 CLINICAL HEMATOLOGY 5 CLINICAL HEMATOLOGY 6 HEMATOPOIESIS 1 All of the cells in the peripheral blood have finite life spans and thus must be renewed continuously. -

Ghid Incepator Al Celulelor Sanguine.Pdf
A BEGINNER’S GUIDE TO BLOOD CELLS A Beginner’s Guide to Blood Cells 2nd Edition Barbara J. Bain MB BS FRACP FRCPath Reader in Diagnostic Haematology, Department of Haematology St Mary’s Hospital Campus, Imperial College, St Mary’s Hospital, London © 1996, 2004 by Blackwell Publishing Ltd Blackwell Publishing, Inc., 350 Main Street, Malden, Massachusetts 02148-5020, USA Blackwell Publishing Ltd, 9600 Garsington Road, Oxford OX4 2DQ, UK Blackwell Publishing Asia Pty Ltd, 550 Swanston Street, Carlton, Victoria 3053, Australia The right of the Author to be identified as the Author of this Work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. First published 1996 Second edition 2004 Library of Congress Cataloging-in-Publication Data Bain, Barbara J. A beginner’s guide to blood cells / Barbara J. Bain. – 2nd ed. p. ; cm. Includes index. ISBN 1-4051-2175-0 1. Hematology–Handbooks, manuals, etc. 2. Blood cell–Handbooks, manuals, etc. [DNLM: 1. Blood Cells–physiology–Handbooks. 2. Blood Cells Count–methods– Handbooks. 3. Blood Cells–pathology–Handbooks. WH 39 B 162b 2004] I. Title. RB45.B268 2004 616.1¢5–dc22 2004001756 ISBN 1-4051-2175-0 A catalogue record for this title is available from the British Library Set in 9.5 on 13 pt Trump by SNP Best-set Typesetter Ltd., Hong Kong Printed and bound in India by Replica Press Pvt. -

Developing a Computer-Based Information System to Improve the Diagnosis of Blood Anemia
I I Developing a Computer-based Information System to Improve the Diagnosis of Blood Anemia By Bashar Abdallah Issa Khawaldeh Supervisor Dr. Basim Alhadidi This Thesis is submitted to the Department of Computer Information Systems, Faculty of Information Technology, Middle East University in partial fulfillment for the requirements for the degree of Master Degree in Computer Information System. Department of Computer Information Systems Faculty of Information Technology Middle East University (May 201 3) Amman – Jordan II III IV V VI ACKNOWLEDGMENTS I would like to thank my supervisor Dr. Basim Alhadidi for his support, encouragement, proofreading of thesis drafts, and helping me throughout my thesis, and so directing to the right track of Image processing. I thank the Information Technology Faculty members at the Middle East University for Graduate Studies; I thank my father and my mother for their continued support during my study. VII DEDICATION All praise belongs to Allah and all thanks to Allah. I dedicate this work to Parents, brothers, sisters, relatives, friends, and to all those who helped, supported and taught me. VIII Table of Contents Developing a Computer- based Information System to Improve the Diagnosis of Blood Anemia .…. I ………………………………….……..…................... .. ...... ………………...………………………..…….………. II Authorization Statement ………………………………………………….…………...………………………...…..…….……. III Examination Committee Decision ………………..…………………...…………………………………...……...…..…... IV Declaration ………………………………………………………………………………………………………………………….... -

Namib La University of Science and Technology
nAmIB lA UnIVERSITY OF SCIEnCE AnD TECHnOLOGY FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF HEALTH SCIENCES QUALIFICATION: BACHELOR OF BIOMEDICAL SCIENCES QUALIFICATION CODE: SOBBMS LEVEL: 6 COURSE CODE: HAM210S COURSE NAME: HAEMATOLOGY 1 SESSION: JUNE 2016 PAPER: THEORY DURATION: 3 HOURS MARKS: 100 FIRST OPPORTUNITY EXAMINATION QUESTION PAPER EXAMINER($) MR.MARTIN GONZO MODERATOR: MR. MUNYARADZI MUKESI INSTRUCTIONS 1. Answer ALL the questions. 2. Write clearly and neatly. 3. Number the answers clearly. PERMISSIBLE MATERIALS THIS QUESTION PAPER CONSISTS OF 7 PAGES (Including this front page} SECTION A [25] MULTIPLE CHOICE QUESTIONS QUESTION 1 [20] Answer ALL questions. Each correct answer is worth ONE mark. 1.1. Which of the following is TRUE for haemopoiesis? (1) a) In the first few weeks of development the long bones are the main site of haemopoiesis b) In adult life all the bone marrow is haemopoietic c) During childhood there is progressive fatty replacement of ma rrow in the long bones d) In adult life haemopoietic marrow is confined to the central skeleton only e) Red marrow forms only red blood cells 1.2. A full blood count was done and the results were as follow: red blood cells 2.86 X1012/L, red cell distribution width 23% and the mean cell volume 65 fl. Select the expected red blood cell morphologies for the above results: (1) a) Severe anisocytosis and spherocytosis b) Howell-Jolly bodies and anisocytosis c) Microcytes with anisocytosis d) Microcytosis with normal size red blood cells e) Microcytosis and target cells 1.3. Juvenile red blood cells are reticulocytes. -

Hematology & Oncology
First Aid Express 2016 workbook: HEMATOLOGY & ONCOLOGY page 1 Hematology & Oncology How to Use the Workbook with the Videos Using this table as a guide, read the Facts in First Aid for the USMLE Step 1 2016, watch the corresponding First Aid Express 2016 videos, and then answer the workbook questions. Facts in First Aid for Corresponding First Aid Workbook the USMLE Step 1 2016 Express 2016 videos questions 378.1–381.1 Anatomy (2 videos) 1–10 381.2–385.2 Physiology (2 videos) 11–14 386.1–404.2 Pathology (9 videos) 15–30 405.1–413.4 Pharmacology (3 videos) 31–35 Copyright © 2016 by MedIQ Learning, LLC All rights reserved v1.0 page 2 First Aid Express 2016 workbook: HEMATOLOGY & ONCOLOGY Questions ANATOMY 1. Define the following terms. (p 378) A. Anisocytosis _______________________________________________________________ B. Poikilocytosis _______________________________________________________________ C. Thrombocytopenia __________________________________________________________ 2. What do the dense granules of platelets contain? (p 378) ________________________________ 3. What do the α-granules of platelets contain? (p 378) ____________________________________ 4. List the types of white blood cells in order of decreasing prevalence. (pp 378) ________________ ______________________________________________________________________________ 5. What conditions can cause hypersegmentation of neutrophils? (p 378) _____________________ ______________________________________________________________________________ 6. CD14 is a cell surface marker -

Pathology Course
Pathology Course Kindly Sponsored by: Edited by: Michelle Kunc, Jhia Teh, Sally Barker and Yvonne Tsitsiou 1 Introduction The Medical Education Society (MedED) was established in 2004 by three students who were keen to develop schemes whereby senior students tutor younger ones - ‘peer-to- peer’ learning. It was decided that teaching would be outside the formal curriculum and the topics covered would reflect learning needs identified by members of the society and student body. This year we have coordinated PACES and Pathology revision courses, which are being delivered by past ICSM students. We hope you enjoy our Year 5 events and find their content useful for your revision. We would like to thank all the doctors involved in the production of this guide for their support and for taking time out of the schedules to come back and teach us. We would also like to thank the previous MedED guide editors: • 2016-2017: Daniel Campioni-Norman, Rhys Smith, Helen-Cara Younan and Rebekah Judge • 2017-2018: Charlie Caird, Stephanie EzeKwe, Mohammad Fallaha, Samyukta Sundar • 2018-2019: Sophia von Widekind, Lasith Ranasinghe, Daniel Huddart, Alex Huddart If you have any questions please contact us at [email protected]. Please note: MedED does not represent the ICSM Faculty or Student Union. This guide has been produced by students and the Pathology Course lecturers. We have made every effort to ensure that the following information is accurate and reliable. However, this guide should not be used to replace formal ICSM teaching and education -
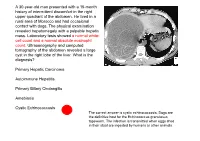
A 30-Year-Old Man Presented with a 15-Month History of Intermittent Discomfort in the Right Upper Quadrant of the Abdomen
A 30-year-old man presented with a 15-month history of intermittent discomfort in the right upper quadrant of the abdomen. He lived in a rural area of Morocco and had occasional contact with dogs. The physical examination revealed hepatomegaly with a palpable hepatic mass. Laboratory tests showed a normal white- cell count and a normal absolute eosinophil count. Ultrasonography and computed tomography of the abdomen revealed a large cyst in the right lobe of the liver. What is the diagnosis? Primary Hepatic Carcinoma Autoimmune Hepatitis Primary Biliary Cholangitis Amebiasis Cystic Echinococcosis The correct answer is cystic echinococcosis. Dogs are the definitive host for the Echinococcus granulosus tapeworm. The infection is transmitted when eggs shed in their stool are ingested by humans or other animals. Die Amöbenruhr (Amöbiasis) ist eine Infektion des Darmes mit dem 1883 von Stephanos Kartulis entdeckten Erreger Entamoeba histolytica. Sie wird von einer Amöbe (Wechseltierchen) der Art Entamoeba histolytica verursacht. Die Krankheitserreger leben im Nahrungsbrei des menschlichen Dickdarms und vermehren sich ungeschlechtlich durch Zellteilung. Als sehr widerstandsfähige Dauerformen kann der Erreger Zysten bilden und in dieser Form im Dickdarm verbleiben – unter Umständen jahrelang ohne jegliche KrankheitsanZeichen Zu verursachen – und wird auch mit dem Stuhl ausgeschieden („Minutaform“). Der InfiZierte ist also gleichZeitig Überträger. Die ausgeschiedene Dauerform kann in der Außenwelt monatelang infektiös bleiben. Aus noch unbekannten Gründen können sich diese Erreger verändern, man kann dann eine veränderte DNA und ein verändertes EnZymmuster beobachten. Durch verschiedene Pathogenitätsfaktoren der „Magnaform“ (pathogene Form abZugrenZen von der Minutaform, die nicht zum Bild der Amöbenruhr führt), Amobaphore, porenbildende EnZyme sowie Zysteinproteasen, die die extraZelluläre Matrix des Colons ZersetZen, kommt es Zu sogenannten geschwulstartigen Amöbom. -

PERIPHERAL BLOOD FILM - a REVIEW AS Adewoyin1 and B
FEATURE ARTICLES Ann Ibd. Pg. Med 2014. Vol.12, No.2 71-79 PERIPHERAL BLOOD FILM - A REVIEW AS Adewoyin1 and B. Nwogoh2 1. Dept. of Haematology & Blood Transfusion, University of Benin Teaching Hospital, Benin City, Edo State 2. Dept. of Haematology & Blood Transfusion, University of Calabar Teaching Hospital, Calabar, Cross River State Correspondence: ABSTRACT Dr. A.S. Adewoyin The peripheral blood film (PBF) is a laboratory work-up Dept. of Haematology and Blood Transfusion, that involves cytology of peripheral blood cells smeared University of Benin Teaching Hospital, on a slide. As basic as it is, PBF is invaluable in the PMB 1111, characterization of various clinical diseases. This article Benin City, Edo State highlights the basic science and art behind the PBF. It E – Mail: [email protected], expounds its laboratory applications, clinical indications Phone: 07033966347 and interpretations in the light of various clinical diseases. Despite advances in haematology automation and application of molecular techniques, the PBF has remained a very important diagnostic test to the haematologist. A good quality smear, thorough examination and proper interpretation in line with patient’s clinical state should be ensured by the haemato-pathologist. Clinicians should be abreast with its clinical utility and proper application of the reports in the management of patients. Keywords: Peripheral blood smear, Preparation, Examination, Interpretation, Reporting, Blood cells morphology. INTRODUCTION In patient care, diagnostic formulations rest -

Peripheral Blood Smear Examination
Board Review- Part 1: Benign HemePath Peripheral Blood Smear Examination Elevated MCV = Macrocytosis MCV > 100um3 • B12/Folate deficiency, aplastic anemia, MDS • Autoimmune hemolytic anemia • Liver disease, hypothyroidism, alcoholism • Cold agglutinin disease Decreased MCV = Microcytosis MCV < 80um3 • Iron deficiency • Thalassemias • Anemia of chronic disease • Hemoglobinopathies – C, E, S, D Iron Panel Interpretation Cause of Serum TIBC Percent anemia iron saturation Iron ↓ ↑ ↓ deficiency Thalassemias ↑ / N ↓ / N ↑ / N Sideroblastic ↑ ↓ / N ↑ anemia Chronic N/↓ ↓ N disease Pathologic Red Blood Cells in Peripheral Blood Smears Type of Cell Underlying Change Disease States Acanthocyte (spur cell) Altered cell membrane lipids Abetalipoproteinemia, liver disease, postsplenectomy, McLeod phenotype Bite Cell (degmacyte) Heinz body pitting by spleen G6PD deficiency, drug-induced oxidant hemolysis Ovalocyte (elliptocyte) Abnormal cytoskeletal proteins Hereditary elliptocytosis Rouleaux Circulating paraprotein Paraproteinemia Schistocyte (helmet cell) Mechanical destruction in DIC, TTP, HUS, prosthetic heart microvasculature valves Spherocyte Decreased membrane Hereditary sphereocytosis, redundancy immunohemolytic anemia (warm Ab) Stomatocyte Membrane defect with Hereditary stomatocytosis, liver abnormal cation permeation disease Target Cell (codocyte) Increased redundancy of cell Liver disease, beta thalassemia membrane postsplenectomy, Hgb C/D/E/S Burr Cell (ecchinocyte) Altered membrane lipids Usually artifactual but maybe uremia Tear Drop -

Peripheral Blood Smear
PERIPHERAL BLOOD FILM EVALUATION WHAT LIES BENEATH? งานประชุมวชิ าการ คณะสัตวแพทยศาสตร์ มหาวิทยาลัยเชียงใหม่ 2563 Multi Systemic Disease Nawin Manachai (DVM., MSc., PhD.) Small Animal Clinic Department of Companion Animal and Wildlife Clinic Faculty of Veterinary Medicine Chiang Mai University • คำถำม ? ในช่วง 6 เดอื นทผี่ ่ำนมำท่ำนดู blood smear บ่อยแค่ไหน ? 1. อย่ำงน้อย 1 ครงั้ ตอ่ สปั ดำห ์ 2. อย่ำงน้อย 1 ครงั้ ตอ่ เดอื น 3. อย่ำงน้อย 1 ครงั้ ตอ่ 3 เดือน 4. อย่ำงน้อย 1 ครงั้ ตอ่ 6 เดือน 5.ไม่เคยดูเลย Peripheral blood smear (PBS) Screening Diagnosis • Hematological disorders • simply -anemia • safe -leukopenia -thrombocytopenia -unexplained cytosis -malignancies • Non-hematological disorders (hematologic manifestations in Early management Monitoring systemic disease) • Peripheral blood smear (PBS) Iron deficiency IMHA Megaloblastic anemia ITP Myelophthisis blood picture MAHA blood picture Hematologic malignancy Blood parasite infection What is included in a complete blood count (CBC) ? Scatter plot data Analyzer data Blood film microscopic review Provided by automated analyzers Provided by automated analyzers 5 Peripheral blood smear (PBS) 1. EDTA-blood 2. Glass slide 3. Coverslip 4. Fixative agent 5. Staining • Wright’s stain • Diff-quick 6. Light microscope 7. You Standard area… stacked RBCs on standard area Advantage zone of morphology Verify automate analyzer results Identify critical diagnostic features that analyzers cannot evaluate Identify morphologic abnormalities can be present even in patients with quantitatively normal results for all Peripheral blood film (smear) feathered edge hematologic parameters Make blood smears soon after collection to reduce the risk of artifacts Make a good quality smear 10X Always start from LOW POWER 10X 1. RBC distribution • degree of anemia • rouleaux formation • autoagglutination 2. WBC estimated number • 10-15 cell/LPF approximate to normal 3.