Conservation Introduction Plan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proposal to Construct and Operate a Satellite Launching Facility on Christmas Island
Environment Assessment Report PROPOSAL TO CONSTRUCT AND OPERATE A SATELLITE LAUNCHING FACILITY ON CHRISTMAS ISLAND Environment Assessment Branch 2 May 2000 Christmas Island Satellite Launch Facility Proposal Environment Assessment Report - Environment Assessment Branch – May 2000 3 Table of Contents 1 INTRODUCTION..............................................................................................6 1.1 GENERAL ...........................................................................................................6 1.2 ENVIRONMENT ASSESSMENT............................................................................7 1.3 THE ASSESSMENT PROCESS ...............................................................................7 1.4 MAJOR ISSUES RAISED DURING THE PUBLIC COMMENT PERIOD ON THE DRAFT EIS .................................................................................................................9 1.4.1 Socio-economic......................................................................................10 1.4.2 Biodiversity............................................................................................10 1.4.3 Roads and infrastructure .....................................................................11 1.4.4 Other.......................................................................................................12 2 NEED FOR THE PROJECT AND KEY ALTERNATIVES ......................14 2.1 NEED FOR THE PROJECT ..................................................................................14 2.2 KEY -

Christmas Island Getaway
Christmas Island Getaway www.ditravel.com.au 1300 813 391 Christmas Island Fly-Drive-Stay Duration: 8 days Departs: daily Stay: 7 nights apartment or lodge Travel style: Independent self-drive Booking code: CHRFDS8AZ Call DI Travel on 1300 813 391 Email [email protected] 8 Days Christmas Island Fly-Drive-Stay Getaway About the holiday Christmas Island is an Australian territory in the Indian Ocean, lying south of Java, Indonesia, and 2600 kilometres north west of Perth, Western Australia. What this small, rocky island lacks in size, it more than makes up for with its extraordinary wildlife and spectacular natural wonders! Known as the ‘Galapagos of the Indian Ocean’, Christmas Island is a haven for animals and sea creatures, with close to two-thirds of the island being a protected national park. The island is famous for its red crabs, sea birds, whale sharks and incredible coral reefs. The island’s close proximity to Asia also means there’s a wonderful blend of cultural influences. So there really are few places on our planet like this remote Australian island! Why you’ll love this trip… Indulge in an Australian island escape that’s unlike any other! Dive some of the world’s longest drop-offs & bathe beneath a rainforest waterfall Time it right to witness the amazing annual crab migration at the start of the wet season! Travel Dates Departs regularly* 2021 – 20 April to 15 June, 20 July to 15 September, 10 October to 10 December 2022 – 25 January to 31 March, 20 April to 15 June, 20 July to 15 September, 10 October to 10 December *Departures are subject to confirmation at time of booking. -

Introduction 3
1 ,QWURGXFWLRQ The inquiry process 1.1 On 8 November 2000 the Senate referred matters relating to the tender process for the sale of the Christmas Island Casino and Resort to the Joint Standing Committee on the National Capital and External Territories, for inquiry and report by 5 April 2001. The reporting date was subsequently extended to 27 September 2001. The full terms of reference are set out at the beginning of this report. 1.2 The inquiry was advertised in the Territories’ Tattler on 1 December 2000 and nationally in The Australian on 6 December 2000. The Committee also wrote to relevant Commonwealth Departments and to a number of organisations, inviting submissions. 1.3 The Committee received fifteen submissions, which are listed at Appendix A, and eleven exhibits, listed at Appendix B. Submissions are available from the Committee’s web site at: www.aph.gov.au/house/committee/ncet 1.4 The Committee held public hearings in Canberra in February and June 2001, and in Perth and Christmas Island in April 2001. Details are listed at Appendix C. Structure of the report 1.5 This report is divided into six chapters. Chapter One provides a background to the inquiry and details on the social, political and economic framework of the Island; 2 RISKY BUSINESS Chapter Two details the history and operation of the Christmas Island Casino and Resort, from its opening in 1993 to its closure in 1998; Chapter Three details the tender and sale process of the casino and resort; Chapter Four examines the conduct of the tender process; Chapter Five examines the outcome of the sale of the casino and resort; and Chapter Six details a number of broader community concerns which formed the context of the inquiry. -

Risky Business
The Parliament of the Commonwealth of Australia 5LVN\%XVLQHVV Inquiry into the tender process followed in the sale of the Christmas Island Casino and Resort Joint Standing Committee on the National Capital and External Territories September 2001 Canberra © Commonwealth of Australia 2001 ISBN 0 642 78402 7 &RQWHQWV Foreword..............................................................................................................................................vii Membership of the Committee..............................................................................................................ix Terms of reference ...............................................................................................................................xi List of abbreviations............................................................................................................................ xiii Overview..............................................................................................................................................xv List of recommendations.....................................................................................................................xix 1 Introduction........................................................................................................... 1 The inquiry process .................................................................................................. 1 Structure of the report .............................................................................................. -

Thesis Final
The University of Notre Dame Australia ResearchOnline@ND Theses 2019 Christmas Island: A question of self-determination Kelvin Matthews Follow this and additional works at: https://researchonline.nd.edu.au/theses Part of the Arts and Humanities Commons COMMONWEALTH OF AUSTRALIA Copyright Regulations 1969 WARNING The material in this communication may be subject to copyright under the Act. Any further copying or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice. This dissertation/thesis is brought to you by ResearchOnline@ND. It has been accepted for inclusion in Theses by an authorized administrator of ResearchOnline@ND. For more information, please contact [email protected]. Chapter 2: Historical Background From the beginning of the European settlement of Australia the various Australian colonies began as part of the British colonial empire. While Australia attained Federation as a Commonwealth in 1901, Christmas Island remained as a British administered colony since settlement in the late nineteenth century until 1958 when ‘sovereignty’ of the Territory of Christmas Island was transferred to Australia without any consultative process with the community by either the British or Australian governments at the time. The history of Christmas Island therefore tells three key stories: the first is the economic and social dominance of phosphate mining since the late nineteenth century, which continues to the present day; the second is the relative recent arrival of ‘Australia’ or ‘mainland conditions’ to the Island despite the formal annexure in 1958; and the third is the unique community that has been created, as evidenced by the remarkable cultural and social composition of the community and unusual administrative and institutional arrangements. -

Question Taken on Notice
QUESTION TAKEN ON NOTICE ADDITIONAL ESTIMATES - 25 FEBRUARY 2014 IMMIGRATION AND BORDER PROTECTION PORTFOLIO (AE14/562) PROGRAMME – Internal Product Senator Ludwig (Written) asked: How many buildings (if any) does the Department or agencies or authorities or Government Corporation within each portfolio own or lease? In regards to any building identified, please also detail, the occupancy rate as expressed as a percentage of the building size. If occupancy is identified as less than 100%, for what is the remaining space used? Answer: Occupancy Location rate (%) Reason (if occupancy <100%) Amman 29.1 Other tenants Ankara 27.6 Other tenants Apia 4.9 Other tenants Athens 23.8 Other tenants Auckland 53.8 Other tenants Bangkok 11.6 Other tenants Beijing 16.1 Other tenants Beirut 43.9 Other tenants Belgrade 28.9 Other tenants Berlin 19.8 Other tenants Brasilia 21.4 Other tenants Buenos Aires 16.9 Other tenants Cairo 28.5 Other tenants Colombo 23.7 Other tenants Dhaka 19.8 Other tenants Dili 7.0 Other tenants Dubai 7.1 Other tenants Geneva 1.5 Other tenants Guangzhou 44.2 Other tenants Hanoi 8.6 Other tenants Harare 13.6 Other tenants Ho Chi Minh City 30.7 Other tenants Hong Kong 22.9 Other tenants Islamabad 14.6 Other tenants Jakarta 10.0 Other tenants Kuala Lumpur 8.2 Other tenants London 9.4 Other tenants Madrid 6.5 Other tenants Manila 19.3 Other tenants Mexico City 12.2 Other tenants Moscow 27.4 Other tenants Nairobi 26.8 Other tenants New Delhi 24.9 Other tenants New Delhi 50.6 Other tenants Nuku'alofa 8.0 Other tenants Ottawa 38.3 Other -
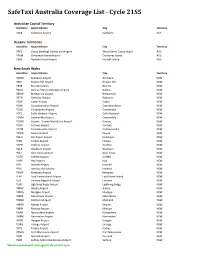
Safetaxi Australia Coverage List - Cycle 21S5
SafeTaxi Australia Coverage List - Cycle 21S5 Australian Capital Territory Identifier Airport Name City Territory YSCB Canberra Airport Canberra ACT Oceanic Territories Identifier Airport Name City Territory YPCC Cocos (Keeling) Islands Intl Airport West Island, Cocos Island AUS YPXM Christmas Island Airport Christmas Island AUS YSNF Norfolk Island Airport Norfolk Island AUS New South Wales Identifier Airport Name City Territory YARM Armidale Airport Armidale NSW YBHI Broken Hill Airport Broken Hill NSW YBKE Bourke Airport Bourke NSW YBNA Ballina / Byron Gateway Airport Ballina NSW YBRW Brewarrina Airport Brewarrina NSW YBTH Bathurst Airport Bathurst NSW YCBA Cobar Airport Cobar NSW YCBB Coonabarabran Airport Coonabarabran NSW YCDO Condobolin Airport Condobolin NSW YCFS Coffs Harbour Airport Coffs Harbour NSW YCNM Coonamble Airport Coonamble NSW YCOM Cooma - Snowy Mountains Airport Cooma NSW YCOR Corowa Airport Corowa NSW YCTM Cootamundra Airport Cootamundra NSW YCWR Cowra Airport Cowra NSW YDLQ Deniliquin Airport Deniliquin NSW YFBS Forbes Airport Forbes NSW YGFN Grafton Airport Grafton NSW YGLB Goulburn Airport Goulburn NSW YGLI Glen Innes Airport Glen Innes NSW YGTH Griffith Airport Griffith NSW YHAY Hay Airport Hay NSW YIVL Inverell Airport Inverell NSW YIVO Ivanhoe Aerodrome Ivanhoe NSW YKMP Kempsey Airport Kempsey NSW YLHI Lord Howe Island Airport Lord Howe Island NSW YLIS Lismore Regional Airport Lismore NSW YLRD Lightning Ridge Airport Lightning Ridge NSW YMAY Albury Airport Albury NSW YMDG Mudgee Airport Mudgee NSW YMER Merimbula -
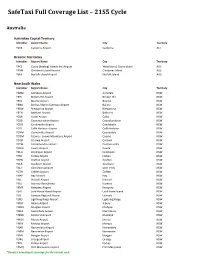
Safetaxi Full Coverage List – 21S5 Cycle
SafeTaxi Full Coverage List – 21S5 Cycle Australia Australian Capital Territory Identifier Airport Name City Territory YSCB Canberra Airport Canberra ACT Oceanic Territories Identifier Airport Name City Territory YPCC Cocos (Keeling) Islands Intl Airport West Island, Cocos Island AUS YPXM Christmas Island Airport Christmas Island AUS YSNF Norfolk Island Airport Norfolk Island AUS New South Wales Identifier Airport Name City Territory YARM Armidale Airport Armidale NSW YBHI Broken Hill Airport Broken Hill NSW YBKE Bourke Airport Bourke NSW YBNA Ballina / Byron Gateway Airport Ballina NSW YBRW Brewarrina Airport Brewarrina NSW YBTH Bathurst Airport Bathurst NSW YCBA Cobar Airport Cobar NSW YCBB Coonabarabran Airport Coonabarabran NSW YCDO Condobolin Airport Condobolin NSW YCFS Coffs Harbour Airport Coffs Harbour NSW YCNM Coonamble Airport Coonamble NSW YCOM Cooma - Snowy Mountains Airport Cooma NSW YCOR Corowa Airport Corowa NSW YCTM Cootamundra Airport Cootamundra NSW YCWR Cowra Airport Cowra NSW YDLQ Deniliquin Airport Deniliquin NSW YFBS Forbes Airport Forbes NSW YGFN Grafton Airport Grafton NSW YGLB Goulburn Airport Goulburn NSW YGLI Glen Innes Airport Glen Innes NSW YGTH Griffith Airport Griffith NSW YHAY Hay Airport Hay NSW YIVL Inverell Airport Inverell NSW YIVO Ivanhoe Aerodrome Ivanhoe NSW YKMP Kempsey Airport Kempsey NSW YLHI Lord Howe Island Airport Lord Howe Island NSW YLIS Lismore Regional Airport Lismore NSW YLRD Lightning Ridge Airport Lightning Ridge NSW YMAY Albury Airport Albury NSW YMDG Mudgee Airport Mudgee NSW YMER -

Documents Released
FOI Rquest FA 20/10/01135 Document 1 OFFICIAL Back Pocket Brie f Numbe r: COO51 BACK POCKET BRIEF Home Affairs Portfolio Department of Home Affairs Budget Estimates – October 2020 BACK POCKET BRIEF Topic: Christmas Island Group: Chie f Ope rating Office r Division: Procurement, Property and Contracts Key Points • Christmas Island Immigration Detention Facilities (IDFs) are comprised of the North West Point Immigration Detention Centre (NWPIDC) and the Phosphate Hill and Construction Camp Alternative Places of Detention (APODs). • On full reactivation, these IDFs provide the capacity to accommodate cohorts of single adult males, females and family groups. • Christmas Island IDFs remain a viable option to supplement the mainland immigration detention network (IDN) capacity. • The operation of NWP as a quarantine facility was not related to onshore immigration detention. Handover of North West Point Immigration Detention Centre as a Quarantine Facility • On 29 January 2020, the Christmas Island Immigration Detention Centre, located at North West Point (NWPIDC), was identified as the location to quarantine Australian citizens evacuated from overseas. 1982 Affairs • This followed the Prime Minister’s announcement that Australian citizens would be Act repatriated from Hubei province in China to a quarantine facility on Christmas Island. • Serco and IHMS, our contracted service providers on Christmas Island, were formally Home advised on or soon after 2 February 2020 that the Department was exercising its Step-in of Rights under the Serco and IHMS Contracts, to effect the handover of NWPIDC to the Commonwealth for control and operation as a quarantine facility. Information of • Christmas Island Maintenance Services (CIMS) our contracted Facilities Maintenance (FM) service provider on Christmas Island was advised on 29 January 2020 of the Prime Minister’s announcement. -

Innovation and Excellence Recognised at AAA National Airport Industry Awards
16 November 2017 Innovation and excellence recognised at AAA National Airport Industry Awards The Australian Airports Association (AAA) hosted its 2017 National Airport Industry Awards last night, recognising innovation and excellence across a range of categories. AAA Chief Executive Officer Caroline Wilkie said the awards showcased the many ways airports are transforming the customer experience for the 154 million passengers travelling through Australia’s airports each year. “Australia’s airports are busier than ever as they invest to deliver a great customer experience for our growing number of passengers,” Ms Wilkie said. “This year’s award winners include Australian airport firsts, significant investments in capacity and innovative new approaches to meeting changing customer needs. “The awards highlight the airport industry’s commitment to investing in our aviation sector to drive growth and build our tourism economy.” Ms Wilkie said the judging panel noted the high standard of submissions, reflecting the wide range of projects and initiatives underway at Australia’s airports. Airport of the Year awards were announced across five categories, with Sydney Airport, Launceston Airport, Coffs Harbour Regional Airport, Tennant Creek Airport and Bendigo Regional Airport taking home the honours. A wide range of Innovation and Excellence Awards were also up for grabs, recognising key projects and initiatives across customer service, commercial, development, operations, environmental management and technology. Sydney Airport Managing Director and CEO Kerrie Mather was also honoured at the event, receiving the Outstanding Contribution to the Airport Industry Award. “All of our winners reflect the passion and dedication of airport staff to lead their industry and create great experiences for the travelling public,” Ms Wilkie said. -

Unleashing Our Tourism Potential
PARLIAMENT OF THE COMMONWEALTH OF AUSTRALIA Northern Horizons – Unleashing Our Tourism Potential Report on the Inquiry into Opportunities and Methods for Stimulating the Tourism Industry in Northern Australia Joint Standing Committee on Northern Australia June 2018 CANBERRA © Commonwealth of Australia ISBN 978-1-74366-661-6 (Printed Version) ISBN 978-1-74366-662-3 (HTML Version) This work is licensed under the Creative Commons Attribution- NonCommercial-NoDerivs 3.0 Australia License. The details of this licence are available on the Creative Commons website: http://creativecommons.org/licenses/by-nc-nd/3.0/au/. Chair's Foreword Northern Australia is home to many iconic locations that attract millions of tourists from across Australia and the world. Uluru in the Northern Territory, the Great Barrier Reef in Queensland, and Broome with its unique pearls in Western Australia are all world-renowned tourism destinations, but only scratch the surface of what Northern Australia’s tourism industry has to offer. In 2014 the Committee’s predecessor, the Joint Select Committee on Northern Australia1, released its report Pivot North: Inquiry into the Development of Northern Australia (Pivot North). Pivot North presented an overarching examination of challenges to, and opportunities for, economic growth and development in Northern Australia. In 2016, the predecessor Committee inquired into opportunities to expand the aquaculture industry. This Committee has now turned its focus to examining ways to stimulate the tourism industry in Northern Australia. The tourism industry presents a major opportunity to support the long term economic and social development of the north and thereby contribute to the sustainability of a large number of remote and regional communities. -

SECTION 1 a Description of the Taxonomy, Biology, Ecology and Life Cycle of the Parasitoid Microhymenopteran Wasp, Tachardiaephagus Somervillei
SECTION 1 A description of the taxonomy, biology, ecology and life cycle of the parasitoid microhymenopteran wasp, Tachardiaephagus somervillei. A. Taxonomy Scientific name Tachardiaephagus somervillei (Mahdihassan, 1923) Common name None Relationships: Tachardiaephagus somervillei (Mahdihassan) is an encyrtid parasitoid: Class: Insecta Order: Hymenoptera Suborder: Apocrita Superfamily: Chalcidoidea Family: Encyrtidae Subfamily: Encyrtinae The Encyrtidae is one of the most important families of parasitic wasps (parasitoids) for the biological control of harmful insects, including a variety of scale insects infesting woody plants (Noyes and Hayat 1994, Noyes 2012). The Encyrtidae currently comprises 460 genera and 3735 species in two subfamilies. The subfamily Encyrtinae includes 353 genera and 2920 species, while the Tetracneminae includes 107 genera and 815 species. Approximately half of all encyrtid species are associated with scale insects (Hemiptera: Coccoidea)(Noyes 2012). Encyrtids are generally endoparasitoids meaning that the parasitoid egg is laid directly inside the host’s body where the hatching larva completes development while feeding on host tissues, ultimately killing the host. Encyrtids mostly parasitize immature life stages (or, rarely, adults), but some species in one genus (Microterys) are egg predators (Noyes 2012). B. Description Tachardiaephagus somervillei was first described by Mahdihassan in 1923 as Lissencyrtus somervilli but Ferrière (1928) transferred L. somervilli Mahdihassan to Tachardiaephagus. He later downgraded this taxon from specific to subspecific rank as T. tachardiae somervilli (Ferrière 1935), based on the presence of intermediate forms and the lack of defining characters. This subspecies was accepted by Mahdihassan (1957). Varshney (1976) emended spelling of the subspecific rank to somervillei. In a recent reconsideration of the taxon, Hayat et al.